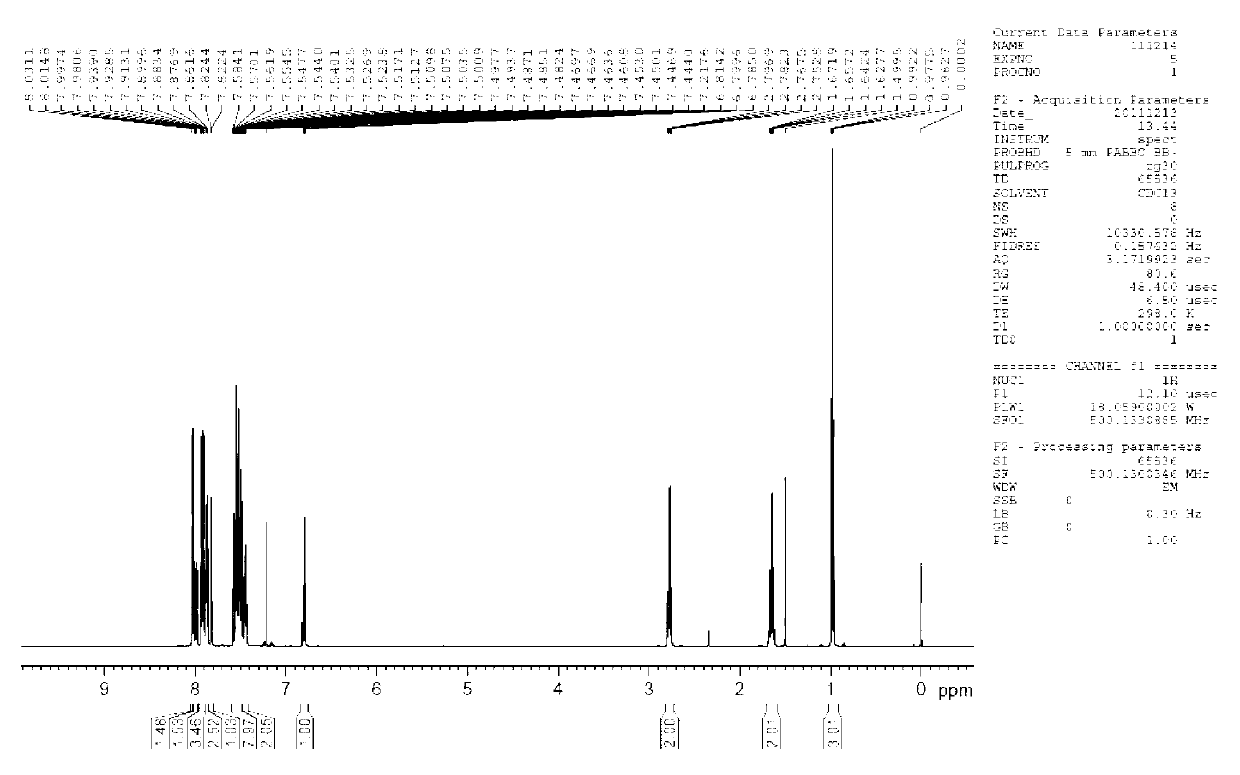
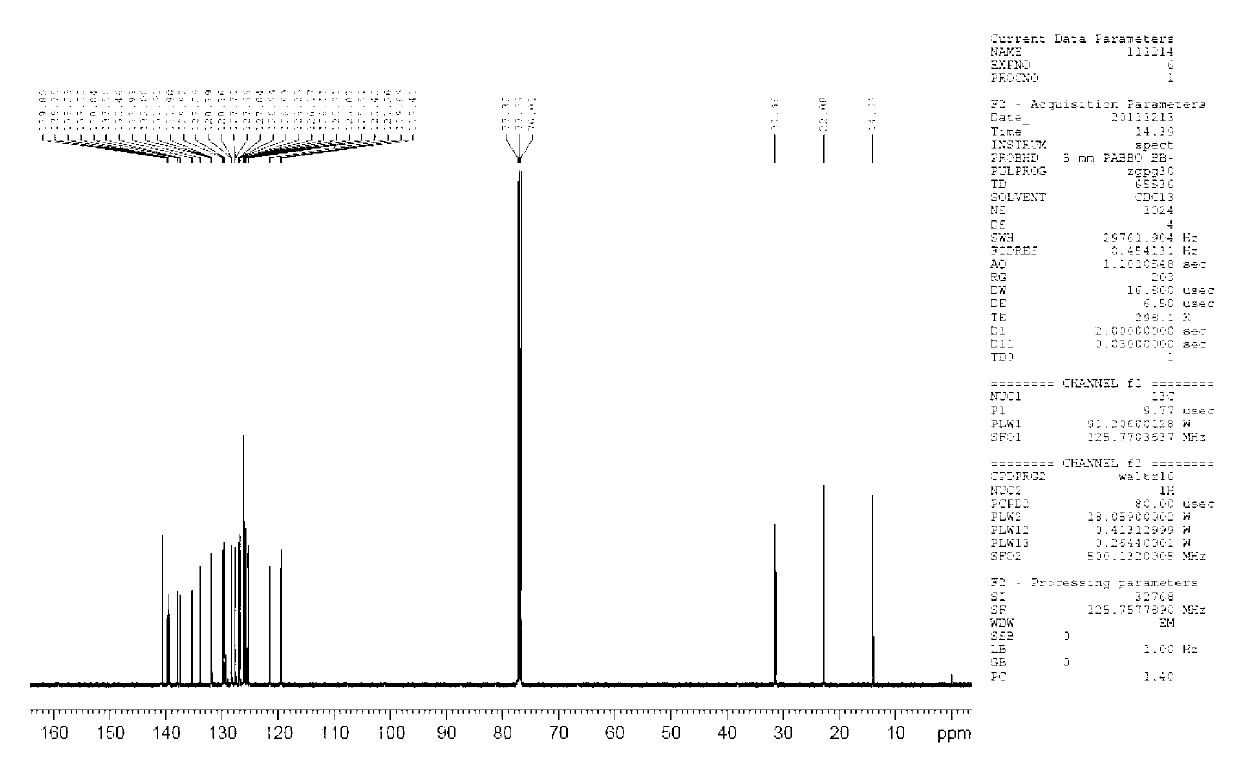
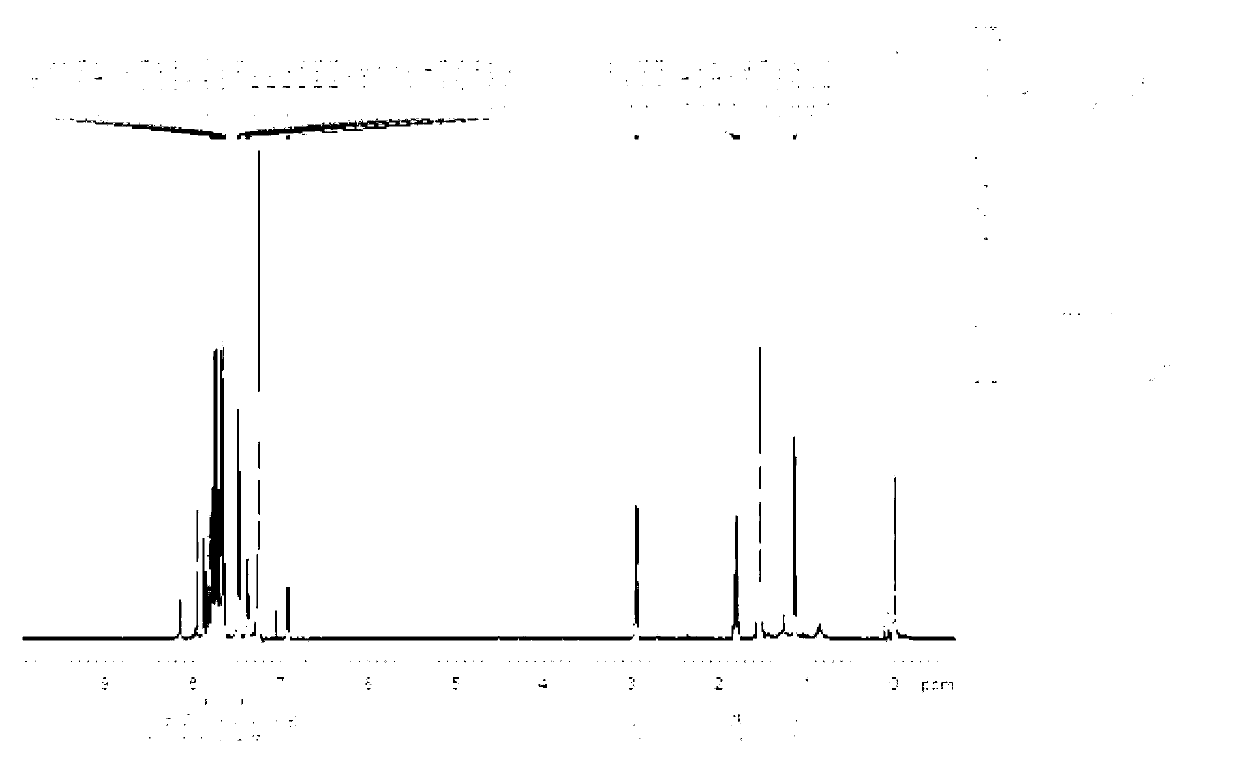
9-alkyl alkenyl-2,7-diaryl fluorene derivate and application of 9-alkyl alkenyl-2,7-diaryl fluorene derivate
A technology of diaryl fluorene and alkyl alkenyl, which is applied in the field of organic optoelectronic materials, can solve the problems affecting the light color purity and stability of devices, and achieves improved glass transition temperature, good thermal stability, and high glass transition. Effects of temperature and decomposition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Synthesis of 9-butylene-2,7-bis(1-naphthyl)fluorene (4,2,7-D(2Np)FBu)
[0045]
[0046] Step 1: Preparation of 2,7-DPNpFK
[0047] Add 2,7-dibromofluorenone (6.8g, 0.02mol), 4-phenyl-1-naphthaleneboronic acid (11.4g, 0.046mol), 74g toluene, 2M potassium carbonate (16.6g, 0.12mol) solution Into the three-necked flask, add Pd (PPh 3 ) 4 (460mg, 0.4mmol), tetrabutylammonium bromide (0.39g, 1.2mmol); Under the protection of nitrogen, react at 85°C for 3hr; after cooling to room temperature, let stand to separate layers, the lower layer is the organic phase, and wash the organic phase with water until Neutral; dried over anhydrous sodium sulfate, filtered; after removing the organic solvent under reduced pressure, beating with methanol; after suction filtration, 11.3 g of yellow particles (the yellow particles were 2,7-DPNpFK) were obtained, with a yield of 96%.
[0048] Step 2: Preparation of 2,7-DPNpFBu-A
[0049] Add 2,7-DPNpFK (5.8g, 0.01mol) and 72gTHF...
Embodiment 2
[0052] Example 2: Synthesis of 9-butylene-2,7-bis(4-biphenyl)fluorene (3,2,7-DBFBu)
[0053]
[0054] Step 1: Preparation of 2,7-DBFK
[0055] Add 2,7-dibromofluorenone (15.0g, 0.04mol), phenylboronic acid (13.0g, 0.11mol), 148g toluene, and 2M potassium carbonate (36.8g, 0.26mol) solution into a three-necked flask to replace nitrogen After adding Pd (PPh 3 ) 4 (920mg, 0.8mmol), tetrabutylammonium bromide (0.78g, 2.4mmol); under the protection of nitrogen, react at 85°C for 3h; after cooling to room temperature, let stand to separate layers, the lower layer is the organic phase, wash the organic phase with water until Neutral; dried over anhydrous sodium sulfate, filtered; after removing the organic solvent under reduced pressure, beating with methanol; after suction filtration, 13.7 g of yellow particles (the yellow particles were 2,7-DBFK) were obtained, with a yield of 93%.
[0056] Step 2: Preparation of 2,7-DBFBu-A
[0057] Add 2,7-DBFK (7.0g, 0.02mol) and 144gTHF ...
Embodiment 3
[0060] Example three: 9-butylene-2,7-bis(4-biphenyl)fluorene (3) photoluminescence spectrum measurement of organic optoelectronic material: 9-butylene-2,7-bis(4-biphenyl) Phenyl) fluorene (3) dubbed 5*10 -5 The dichloromethane dilute solution of M was measured by JV-3150 visible spectrometer and RF-530XPC fluorescence spectrometer, and the photoluminescence spectrum was measured at the maximum absorption wavelength of ultraviolet absorption. The absorption wavelengths of compound 3 are 375nm and 326nm, and the maximum fluorescence emission wavelength is 458nm. (see attached Figure 11 , attached Figure 12 )
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com