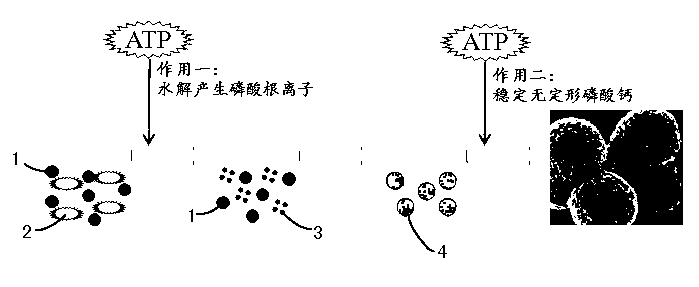
Amorphous calcium phosphate nanoball and preparation method thereof
A technology of amorphous calcium phosphate and nanospheres, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of irregular shape of amorphous calcium phosphate, long operation time, difficult size control, etc. Drug loading capacity, good biodegradability, and the effect of improving biological properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
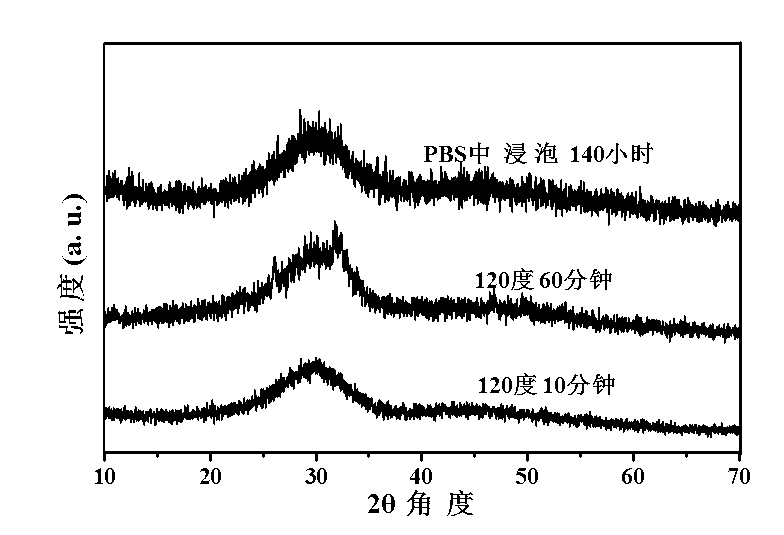
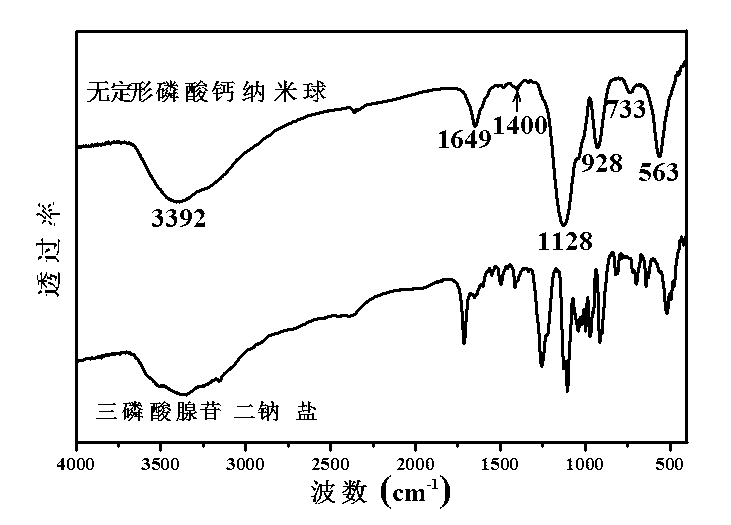
[0046] At room temperature, 0.1470 g of CaCl 2 2H 2 O was dissolved in 30 ml of deionized water to form solution A, and 0.1102 g of adenosine triphosphate disodium salt hydrate was dissolved in 10 ml of deionized water to form solution B. After adjusting liquid A with 1 mol / L hydrochloric acid to make its pH equal to 5, add liquid B dropwise, and use magnetic stirring during this process to keep the pH at about 5. After the dropwise addition was completed, the mixed clear solution was transferred to a microwave reactor (capacity 60 ml) and reacted at 100 °C for 30 min. After the reaction system was naturally cooled to room temperature, the product was taken out and centrifuged. The separated product was washed three times with deionized water, once with absolute ethanol, and dried in air at 60°C to obtain Figure 4 In the shown calcium phosphate nanosphere powder, the average diameter of the amorphous calcium phosphate nanosphere is 260 nanometers.
Embodiment 2
[0048] At room temperature, 0.1470 g of CaCl 2 2H 2 O was dissolved in 30 ml of deionized water to form solution A, and 0.1102 g of adenosine triphosphate disodium salt hydrate was dissolved in 10 ml of deionized water to form solution B. After adjusting liquid A with 1 mol / L hydrochloric acid to make its pH equal to 5, add liquid B dropwise, and use magnetic stirring during this process to keep the pH at about 5. After the dropwise addition was completed, the mixed clear solution was transferred to a microwave reactor (capacity 60 ml) and reacted at 120 °C for 10 min. After the reaction system was naturally cooled to room temperature, the product was taken out and centrifuged. The separated product was washed three times with deionized water, once with absolute ethanol, and dried in air at 60°C to obtain Figure 5 And attached Figure 6 The shown calcium phosphate nanosphere powder, the average diameter of the amorphous calcium phosphate nanosphere is 238 nanometers, see a...
Embodiment 3
[0050] At room temperature, 0.1470 g of CaCl 2 2H 2 O was dissolved in 30 ml of deionized water to form solution A, and 0.1102 g of adenosine triphosphate disodium salt hydrate was dissolved in 10 ml of deionized water to form solution B. After adjusting liquid A with 1 mol / L hydrochloric acid to make its pH equal to 5, add liquid B dropwise, and use magnetic stirring during this process to keep the pH at about 5. After the dropwise addition was completed, the mixed clear solution was transferred into a microwave reactor (capacity 60 ml) and reacted at 120 °C for 30 min. After the reaction system was naturally cooled to room temperature, the product was taken out and centrifuged. The separated product was washed three times with deionized water, once with absolute ethanol, and dried in air at 60°C to obtain Figure 7 In the shown calcium phosphate nanosphere powder, the average diameter of the amorphous calcium phosphate nanosphere is 257 nanometers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com