Synthesis method for stable isotope labeled leucomalachite green
A leuco-malachite green and stable isotope technology, which is used in the preparation of imino compounds, the preparation of amino compounds from amines, organic chemistry, etc. Malachite green and leuco-malachite green, the method is cumbersome and other problems, to achieve good economy and use value, stable atomic utilization, simple method and process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
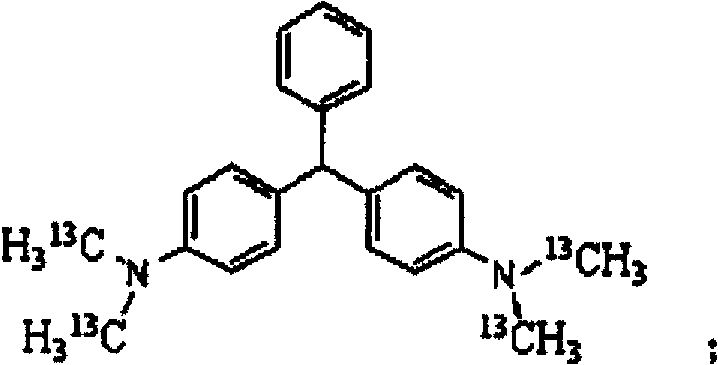
[0029] an isotope 13 The synthetic method of C mark leuco malachite green, this method specifically comprises the following steps:
[0030] the isotope 13 C-labeled N,N-dimethylaniline- 13 C 2 Mix it with benzaldehyde at a molar ratio of 2:1, and with urea at a molar ratio of 2:1, then place it in acetic acid, control the reaction temperature at 90°C, and stir for 6 hours to obtain 13 C-marked leuco malachite green is the product with a chemical purity of 99.1% and an isotopic abundance of 99.2% atom.
[0031] owned 13 The leuco malachite green marked by C can be further oxidized to obtain malachite green, which will 13 C-marked leuco malachite green is placed in a mixed solution of acetic acid and acetic anhydride, and then FeCl is added 3 , FeCl 3 and 13 The weight ratio of C-labeled leuco malachite green is 0.04:1, under the nitrogen and oxygen atmosphere, the reaction temperature is controlled at 70°C, and the reaction is stirred for 8h to obtain 13 C-labeled mala...
Embodiment 2
[0033] an isotope 13 The synthetic method of C mark leuco malachite green, this method specifically comprises the following steps:
[0034] the isotope 13 C-labeled N,N-dimethylaniline- 13 C 2 It is mixed with paraformaldehyde in a molar ratio of 3:1, and benzaldehyde in a molar ratio of 3:1, and then placed in an acetonitrile solution. The reaction temperature is controlled at 120°C, and the reaction is stirred for 4 hours to obtain 13 C-marked leuco malachite green is the product with a chemical purity of 99.0% and an isotopic abundance of 99.3% atom.
[0035] owned 13 The leuco malachite green marked by C can be further oxidized to obtain malachite green, which will 13 C-labeled leuco malachite green is placed in acetone solution, then CuCl is added, CuCl and 13 The weight ratio of C-marked leuco malachite green is 0.1:1, under nitrogen and oxygen atmosphere, the reaction temperature is controlled at 50°C, and the reaction is stirred for 6h to obtain 13 C-labeled mal...
Embodiment 3
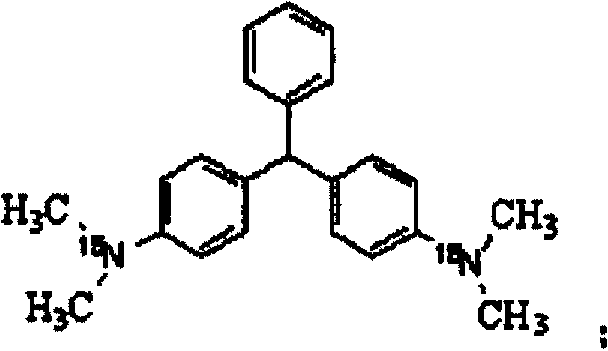
[0037] an isotope 15 The synthetic method of N mark leuco malachite green, this method specifically comprises the following steps:
[0038] the isotope 15 N-labeled N,N-dimethylaniline- 15 The molar ratio of N to benzaldehyde is 3:1, mixed with paraformaldehyde at a molar ratio of 4:1, then placed in acetic anhydride, the reaction temperature is controlled at 160°C, and the reaction is stirred for 4 hours to obtain 15 N-labeled leuco malachite green is the product with a chemical purity of 99.5% and an isotopic abundance of 99.2% atom.
[0039] owned 15 N-labeled leuco malachite green can also be further oxidized to obtain malachite green, which will 15 N-labeled leuco malachite green was placed in acetic acid solution, then CuCl was added, CuCl and 15 The weight ratio of N-labeled leuco malachite green is 0.06:1, under the nitrogen and oxygen atmosphere, the reaction temperature is controlled at 90°C, and the reaction is stirred for 6h to obtain 15 N-labeled malachite gre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com