Phosphor series benzoxazine and preparation method
A technology of benzocyclohexane and oxo, which is applied in the field of phosphorus-based oxonitrobenzocyclohexane and its preparation, can solve the problems of being complicated, inconvenient to operate, unsuitable for large-scale production and the like, and achieve the synthesis steps Simple, easy to process, improve the effect of poor flame resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
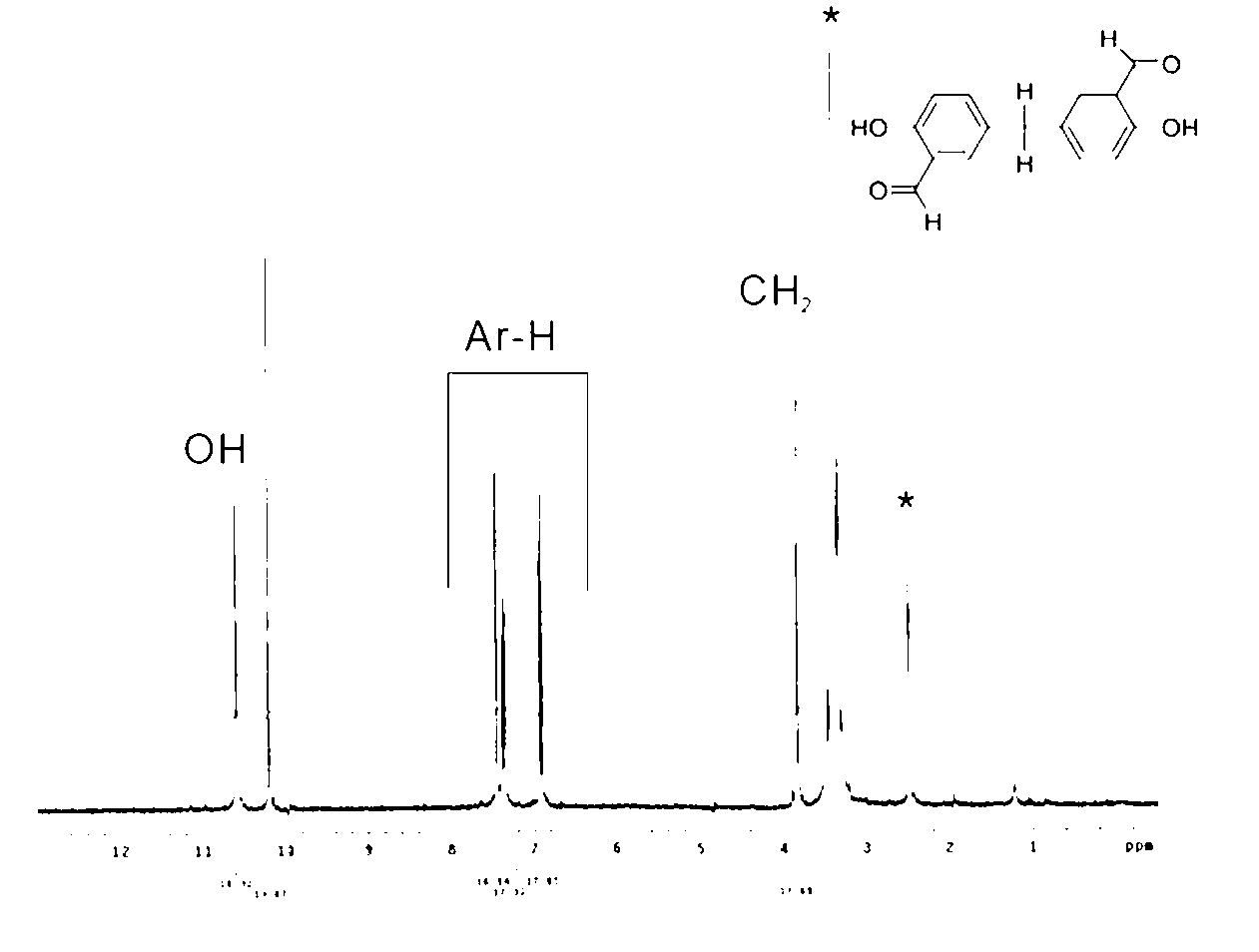
[0028] Embodiment 1.5, the synthesis of 5'-methylene-bis-2-hydroxybenzaldehyde
[0029] 5,5'-methylene-bis-2-hydroxybenzaldehyde is obtained by reacting 2-hydroxybenzaldehyde with trioxane, and its chemical reaction formula is:
[0030]
[0031] Its synthetic steps are as follows:
[0032] In a 0.25L three-neck reactor with a temperature indicating device, add 10g (0.111mol) of trioxane, 89.5g (0.733mol) of 2-hydroxybenzaldehyde, 100g of acetic acid and 3.09g of sulfuric acid React at 85°C for 24 hours. After the reaction, let cool and precipitate naturally. Dissolve the precipitate in methanol and pour it directly into 1000mL deionized water for precipitation and washing, and filter it with a suction filter. The filter cake was dried in a vacuum oven at 110° C. to obtain the product, and the yield was 40%.
[0033] The molecular formula of this product is C 15 h 12 o 4 , the high resolution mass spectrometer identified its molecular weight as 256.2534. Its NMR as fi...
Embodiment 27
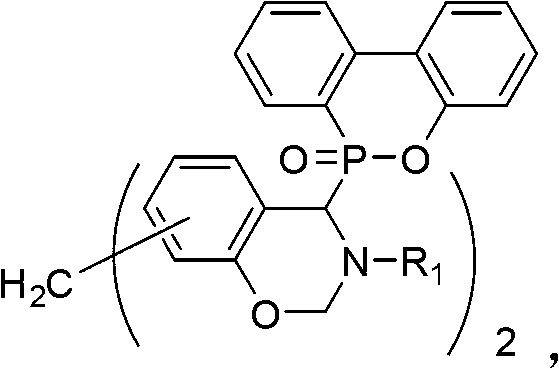
[0034] Example 2.7,7'-methylene-bis-[2-dihydro-3-phenyl-4-(9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) Synthesis of -4-Hydroxy-1,3-Oxoazinobenzocyclohexane] (Bz-a)
[0035] Bz-a is obtained by reacting DOPO, 5,5'-methylene-bis-2-hydroxybenzaldehyde, aniline and formaldehyde, and its chemical reaction formula is:
[0036]
[0037] The synthesis steps are as follows: In a 0.25L three-neck reactor with a temperature indicating device, add 12.81g (0.05mol) of 5,5'-methylene-bis-2-hydroxybenzaldehyde, 9.3g (0.1 mol) of aniline, 100mL of methanol and 21.6g (0.1mol) of DOPO were reacted at room temperature (25°C) for 18 hours, white powder precipitated during the reaction, and 8.9g (0.11mol) was added immediately after the reaction with a volume fraction of 37% Formaldehyde aqueous solution, first react at room temperature (25°C) for 4 hours, then raise the reaction temperature to reflux temperature, react at reflux temperature for 12 hours, after cooling down to room tem...
Embodiment 37
[0038] Example 3.7,7'-methylene-bis-[2-dihydro-3-n-propyl-4-(9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide )-4-Hydroxy-1,3-oxoazinobenzocyclohexane] (Bz-b)
[0039] Bz-b is obtained by reacting DOPO, 5,5'-methylene-bis-2-hydroxybenzaldehyde, propylamine and formaldehyde, and its chemical reaction formula is:
[0040]
[0041] The synthesis steps are as follows: In a 0.25L three-neck reactor with a temperature indicating device, add 12.81g (0.05mol) of 5,5'-methylene-bis-2-hydroxybenzaldehyde, 5.91g (0.1 mol) of propylamine, 100mL of methanol and 21.6g (0.1mol) of DOPO were reacted at room temperature (25°C) for 18 hours. During the reaction, a yellow powder was precipitated. Immediately after the reaction, 8.93g (0.11mol) was added with a volume fraction of 37%. Formaldehyde aqueous solution, first react at room temperature (25°C) for 4 hours, then raise the reaction temperature to reflux temperature, react at reflux temperature for 12 hours, after returning to room t...
PUM
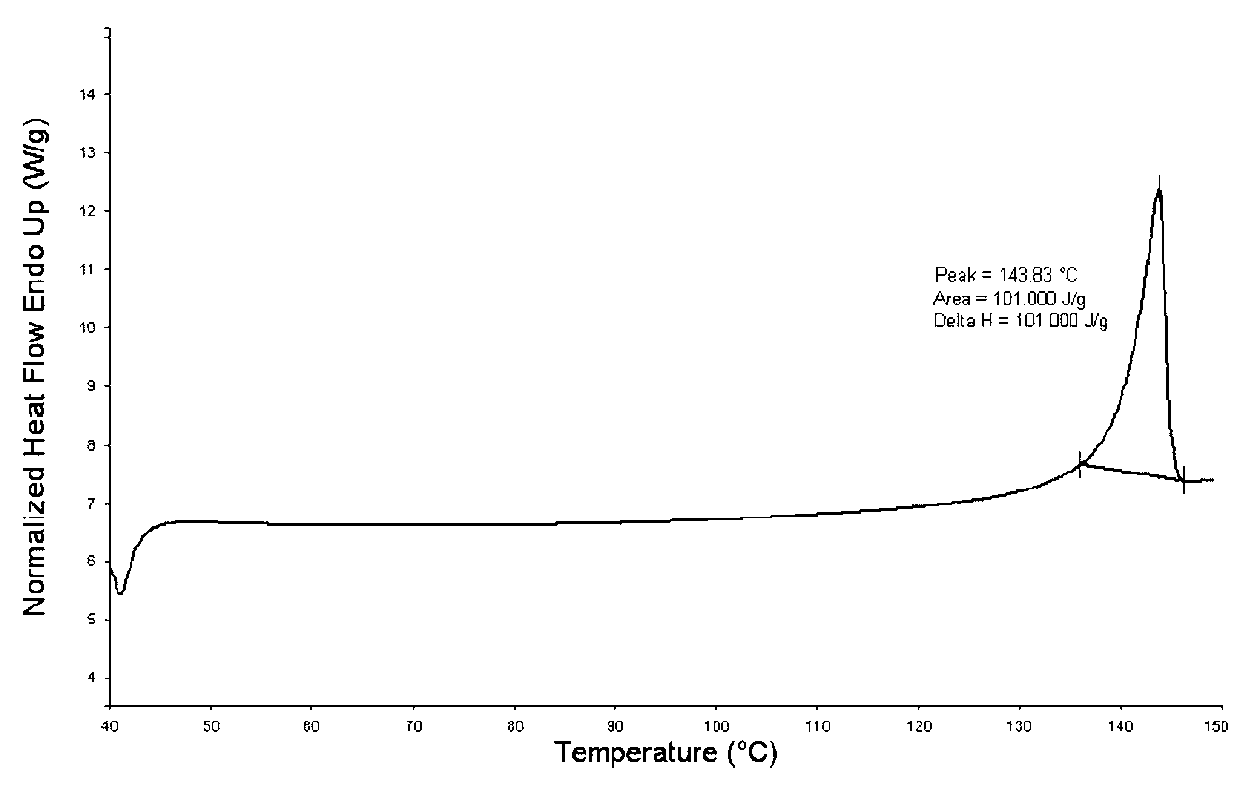
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com