Human glucagon-like peptide-1 analogue
A technology for glucagon and related peptides, applied in the field of genetic engineering, can solve the problems of many steps, low yield and high cost of chemical synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] M1: 5'-ATATGGATCCGCCGCACGGTGAAGGCACCTTTACCTCTGACGTG TCTTCT TACCTG-3', containing BamH I restriction site
[0067] M2: 5'-GACGTGTCTTTCTTACCTGGAAGGCCAGGCTGCTAAAGAATTTATTGCTTGG-3'
[0068] M3: 5'-GGCCAAGCTTAATCACGACCTTTCACCAGCCAAGCAATAAATTCTTT-3', containing HindIII restriction site
[0069] M4: 5'-GGCCAAGCTTAATCACGACCTTTCACCAG-3'
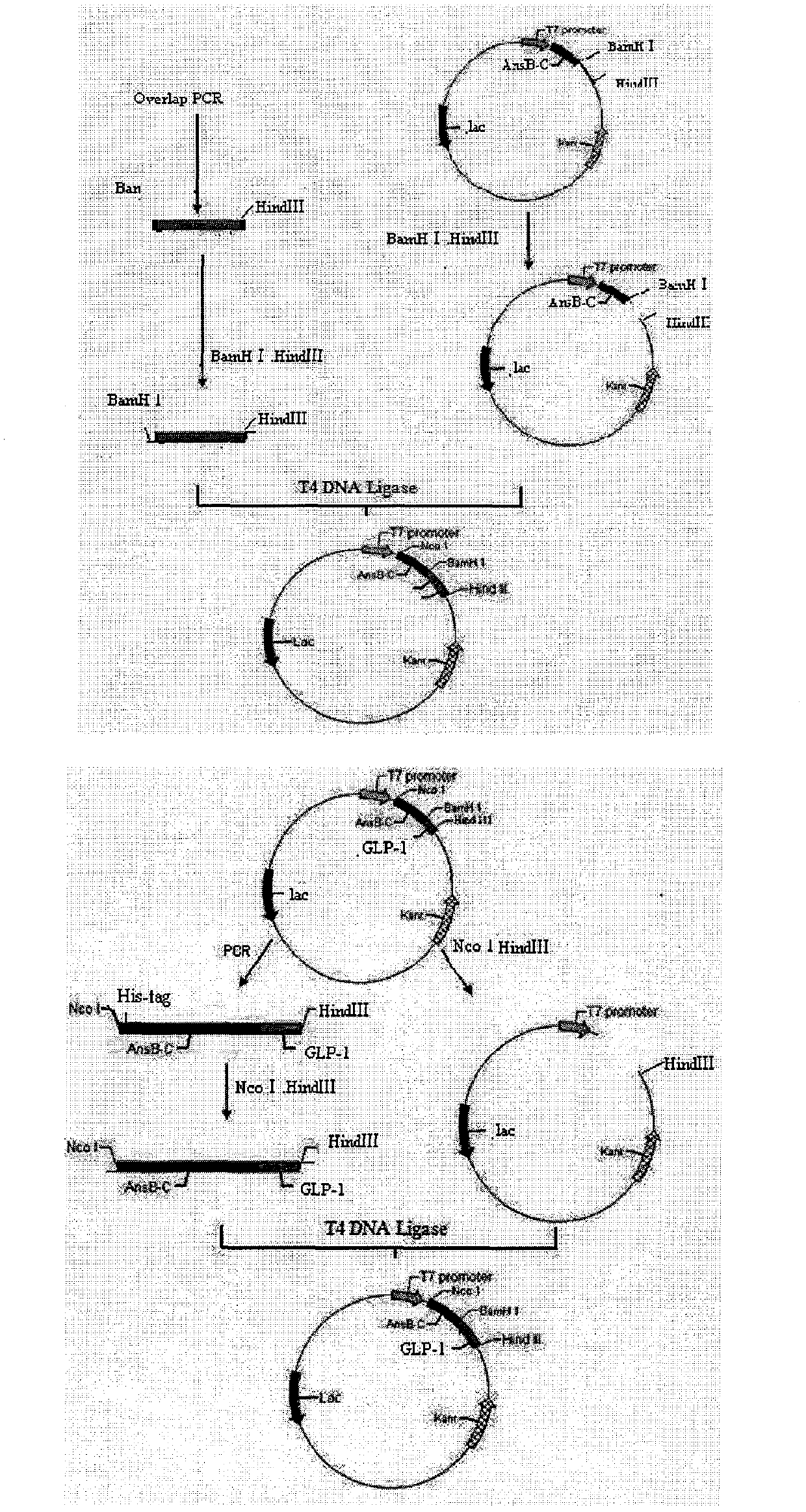
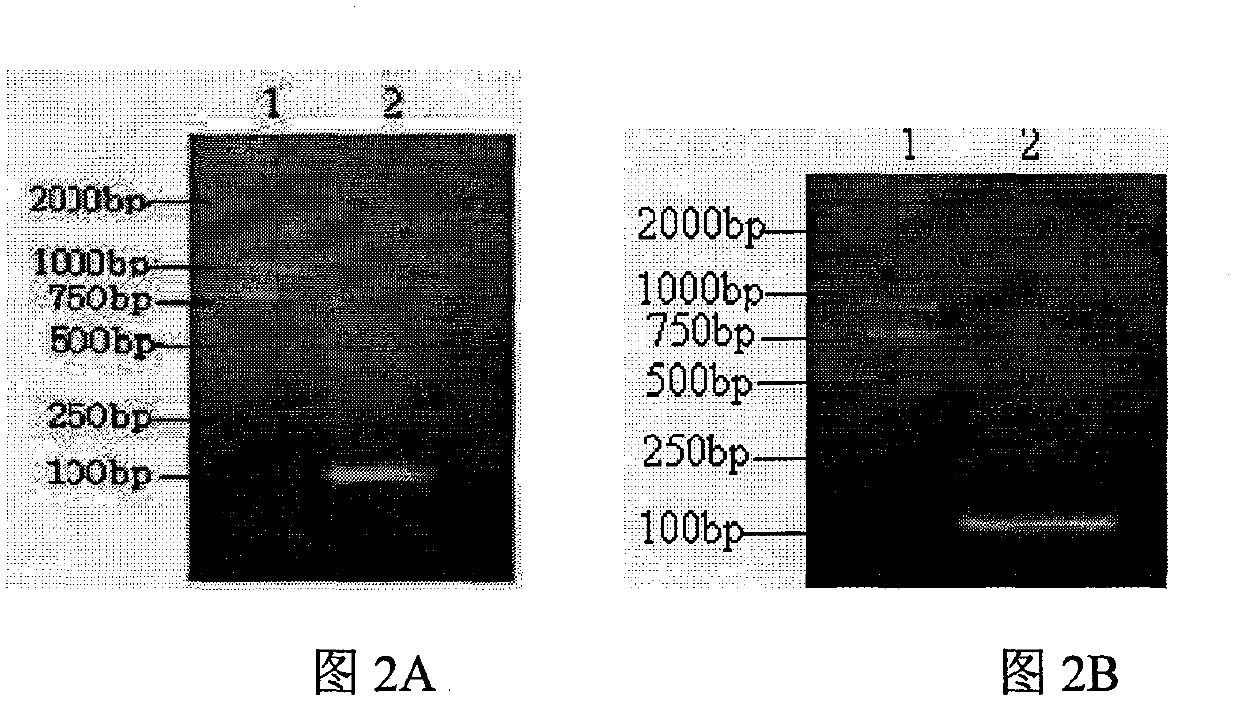
[0070]Synthesized by Shanghai GenScript Biotechnology Co., Ltd. The PCR reaction was carried out on a Bernie PCR amplifier. Among them, M1, M2, and M3 were mixed according to the concentration ratio of 10:1:10, at 94°C for 30s; at 54°C for 60s; at 72°C for 90s; a total of 30 cycles. The PCR products were identified by 1.8% agarose gel electrophoresis, and the target fragments were recovered with a gel recovery kit and stored at -20°C.
[0071] The PCR target fragment and the carrier plasmid pED recovered by the gel were digested by BamH I and HindIII respectively, the target fragment was recovered by agarose gel electrophoresis, ligated ove...
Embodiment 2
[0080] Shake the recombinant expression bacteria in liquid LB medium overnight at 37 degrees, transfer to corn steep liquor fermentation medium with 3% inoculum, culture at 37 degrees for 4 hours, add final concentration of 5 mmol / L lactose to induce expression, collect and ferment after 6-8 hours bacteria. Reserve samples were analyzed by 15% SDS-PAGE. An obvious protein band appeared at the molecular weight of about 17700Da. The fusion protein reached a stable maximum expression level 6h after induction, and BandScan analysis showed that the fusion protein accounted for more than 40% of the total bacterial protein, and formed inclusion bodies in the cell.
[0081] Example 3 Fusion Protein Insertion of His Tag
Embodiment 3
[0082] According to the preferred codon and design requirements of Escherichia coli, 6 histidines were inserted upstream of the fusion protein to form a histidine tag. The His-tag was inserted by PCR, and the upstream and downstream primers were designed:
[0083] Nco I His Up: 5'-TATACCATGGATCATCATCATCATCATACGCCATTCG-3', containing Nco I restriction site
[0084] HindIII down1: 5'-GGCCAAGCTTAATCACGACCTTTCACCAG-3', containing HindIII restriction site
[0085] The plasmid pED-PP-h[Gly8]GLP-1(7-36)D was used as a template, and Nco I His Up and HindIII down1 were used as upstream and downstream primers for PCR amplification. The reaction conditions were the same as in Example 1. The PCR product was digested with Nco I and BamH I, and the plasmid pED-PP-h[Gly8]GLP-1(7-36)D was digested with Nco I and HindIII to remove AnsB-C-PP-h[Gly8] GLP-1(7-36)D gene fragment, the restriction products of the two were ligated to construct the recombinant plasmid pET-His-AnsB-C-pro-pro-h[Gly8]GL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com