Folic acid functionalized drug-loaded mesoporous silica and preparation method thereof
A mesoporous silicon oxide and mesoporous technology, which is applied in the direction of silicon oxide, silicon dioxide, pharmaceutical formulations, etc., can solve problems such as drug leakage, achieve short reaction time, easy operation, and avoid early leakage effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Preparation of folic acid-functionalized drug-loaded mesoporous silica nano-drug carrier material
[0025] 1. Prepare mercapto-modified mesoporous silica by following methods of co-condensation and post-synthesis grafting:
[0026] a) Co-condensation method: put 1g of n-hexadecylammonium bromide (2.8mmol) in a round bottom flask, add deionized water (480mL, 26.7mol) and stir vigorously, add 2M sodium hydroxide (3.5mL, 0.175mmol) to adjust the pH to 11. A mixture of 5 mL tetraethylorthosilicate (22.4 mmol) and 0.97 mL mercaptopropyltrimethoxysilane (5.24 mmol) was added at a temperature of 80° C., and stirring was continued for 2 hours under nitrogen protection. After cooling, the reaction solution was centrifuged to obtain a white gel, which was washed several times with deionized water and ethanol, and dried in vacuum to obtain mercapto-modified mesoporous silica.
[0027] b) Post-synthesis grafting method: put 0.9 g of mercapto-modified mesoporous silica ...
Embodiment 2
[0032] Embodiment 2 drug release test in vitro:
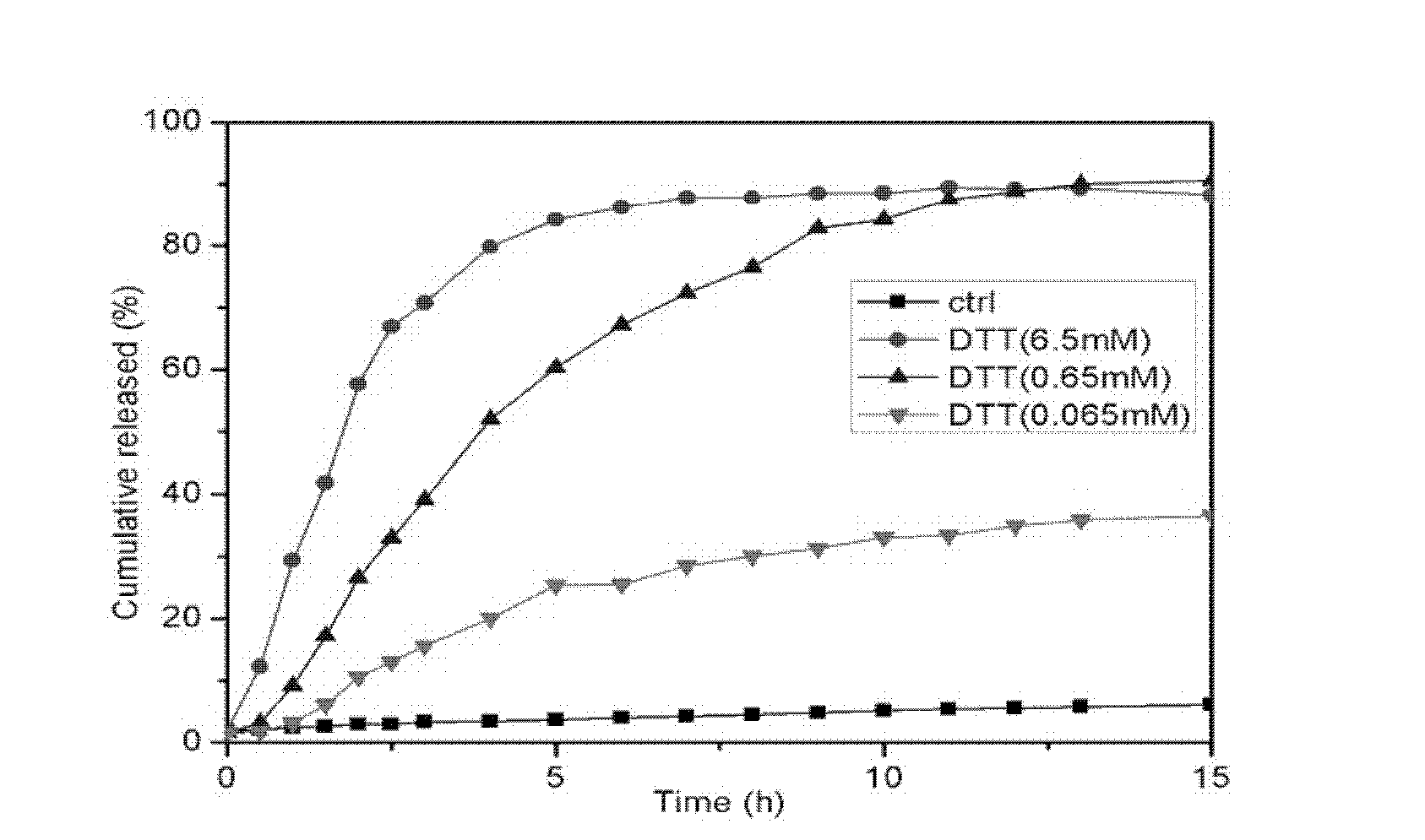
[0033]At 37°C, 1.5 mg of F-MS-F obtained in Example 1 was dispersed in 3 mL of PBS buffer solution, 3.0 mg of DTT (6.5 mM) was added, and a F-2500 fluorescence spectrophotometer (HITACHI, Japan) was used to monitor 515 nm the fluorescence intensity at . Excitation wavelength: 504nm, slit: 2.5nm.
[0034] The reducing agent DTT was not added to the solution of the control group, and the reducing agent DTT of different concentrations was added to the solution of the experimental group. The release curve is drawn with time as the abscissa and cumulative release concentration as the ordinate. From figure 1 It can be seen that the control group did not add DTT, the disulfide bond did not break, the pores were blocked by folic acid, and the model drug was almost not released; while in the experimental group, the drug release rate was uniform due to the addition of DTT, the break of the disulfide bond Faster than the control group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com