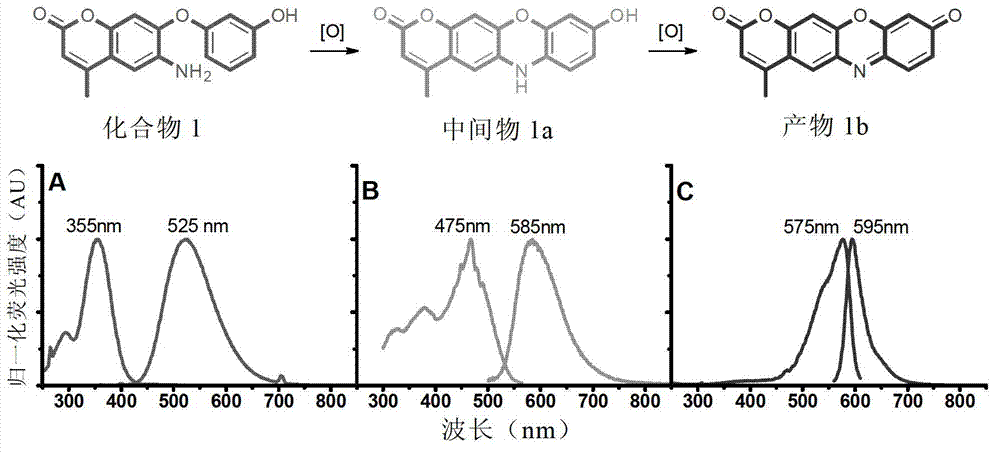
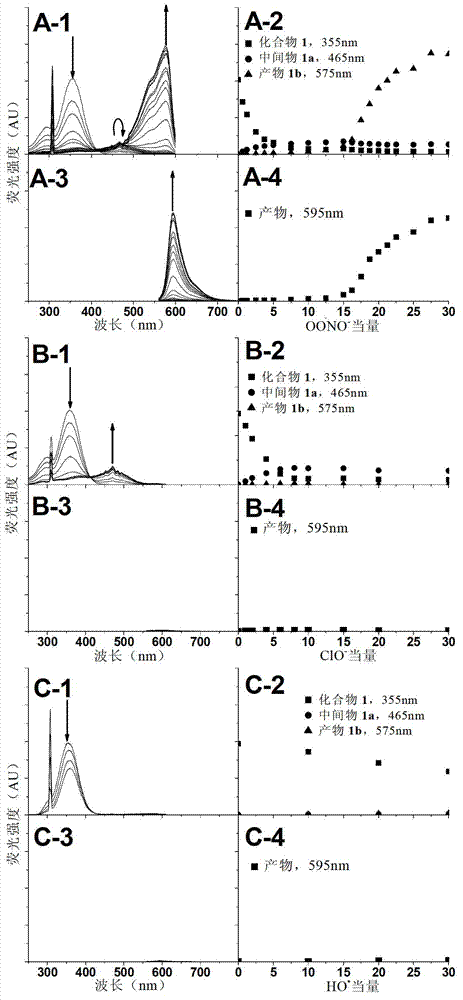
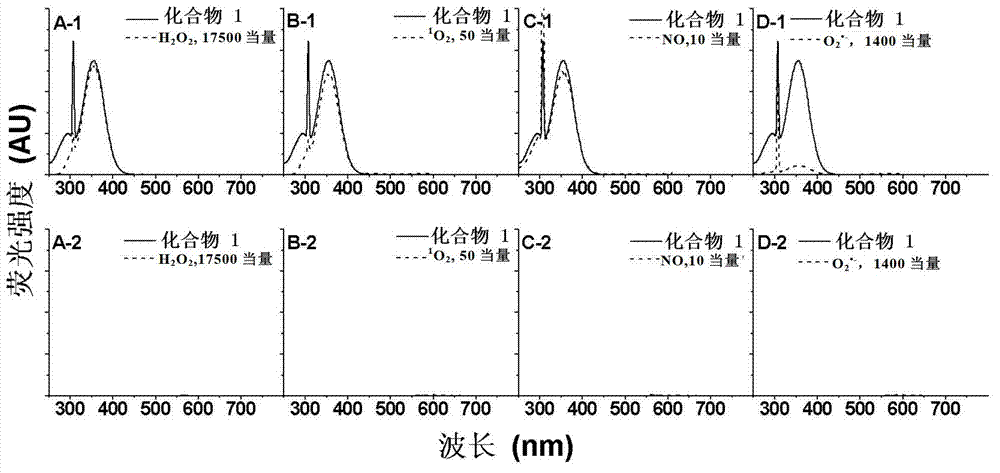
Reagent and method for detecting biological endogenous oxidants
A technology of compound and composition, applied in the detection reagent and field of biological endogenous oxidant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Embodiment 1: the preparation of compound 1
[0129]
[0130] 100mg (0.45mmol) of 6-nitro-7-fluoro-4-methylcoumarin, 56mg of m-methoxyphenol (0.45mmol) and 62mg of potassium carbonate (0.45mmol) were added to 20ml of acetonitrile, reflux Stir overnight, then filter with suction, spin the filtrate to dry the solvent under reduced pressure, and separate the product with a chromatographic column, eluting with a mixed solvent of petroleum ether / ethyl acetate (2:1, V / V), to obtain 82 mg of a yellow solid, with a yield of 60 %.
[0131] Take 210 mg (0.64 mmol) of the compound obtained in the previous step into a reaction flask, add a catalytic amount of 5% Pd / C, and methanol as a solvent, put it in a hydrogenation reduction device and react for 6 hours, suction filter after the reaction, and depressurize the filtrate The solvent was spin-dried, and the product was filtered off Pd / C with a very short chromatographic column, and then recrystallized with dichloromethane to o...
Embodiment 2
[0135] Embodiment 2: the preparation of compound 2
[0136]
[0137] 6-nitro-7-fluoro-4-methylcoumarin 650mg (2.91mmol) and 870mg of 4-chloro-3-hydroxy p-toluenesulfonate (2.91mmol) and 415mg of potassium carbonate (3.00mmol ) was added to 40ml of acetonitrile, refluxed and stirred overnight, then suction filtered, the filtrate was spin-dried under reduced pressure, the product was separated by chromatographic column, and the eluent was petroleum ether / ethyl acetate mixed solvent (15:4, V / V) , to obtain 320mg of yellow solid, yield 22%.
[0138] Take 200mg (0.40mmol) of the compound obtained in the previous step into a reaction flask, add a catalytic amount of 5% Pd / C, and methanol as a solvent, put it in a hydrogenation reduction device and react for 6 hours, suction filter after the reaction, and depressurize the filtrate The solvent was spin-dried, and the product was filtered off Pd / C with a very short chromatographic column, and then recrystallized with ethyl acetate / ...
Embodiment 3
[0141] Embodiment 3: the preparation of compound 3
[0142]
[0143] 100mg (0.45mmol) of 6-nitro-7-fluoro-4-methylcoumarin, 56mg of m-methoxyphenol (0.45mmol) and 62mg of potassium carbonate (0.45mmol) were added to 20ml of acetonitrile, reflux Stir overnight, then filter with suction, spin the filtrate to dry the solvent under reduced pressure, the product is separated by a chromatographic column, and the eluent is a mixed solvent of petroleum ether / ethyl acetate (2:1, V / V), and 82 mg of a yellow solid is obtained, with a yield of 60 %.
[0144] Take 210 mg (0.64 mmol) of the compound obtained in the previous step into a reaction flask, add a catalytic amount of 5% Pd / C, and methanol as a solvent, put it in a hydrogenation reduction device and react for 6 hours, suction filter after the reaction, and depressurize the filtrate The solvent was spin-dried, and the product was filtered off Pd / C with a very short chromatographic column, and then recrystallized with dichlorometha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com