proton conducting membrane
A proton-conducting membrane and material technology, which is used in the conversion of alkanes to alkenes, and the application of composite metal oxides in the preparation of proton-conducting membranes, which can solve problems such as moving too fast.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
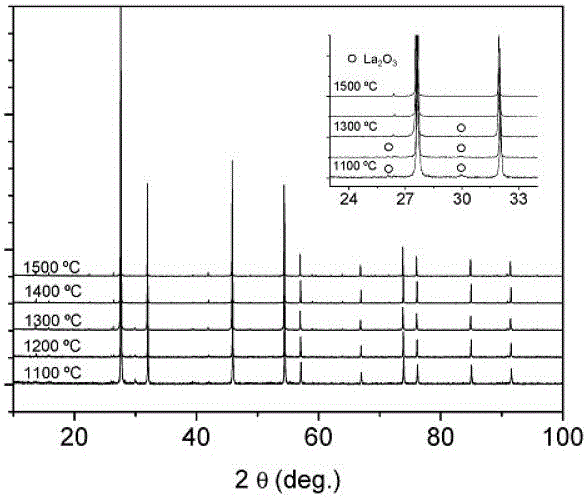
[0231] Oxide powder is prepared by freeze-dried precursor method (Magraso et al. Dalton Transactions, 2009, 10273-10283), and La 2 o 3 and WO 3 Soluble in dilute nitric acid and hydroxide solutions respectively, WO 3 Soluble in aqueous lye to form tungstate ion [WO 4 ] 2+ .
[0232] The ethylenediaminetetraacetic acid was added to each solution as a dispersant, the molar ratio to the ligand metal was 1:1, and the pH value was adjusted to 7~8. After neutralization, the two cation solutions mixed without visible precipitation. Droplets of the clear solution were then flash frozen in liquid nitrogen and lyophilized for 3 days. In this way, the amorphous precursor is prepared, immediately fired at 300°C for 15 minutes to prevent hydration, and then the particles are calcined at 600°C for 2~4 hours until a white powder is formed to ensure that the organic matter in the precursor is completely decomposed . Next, the powder is calcined at 1100~1500°C for 2 hours, and the char...
Embodiment 2
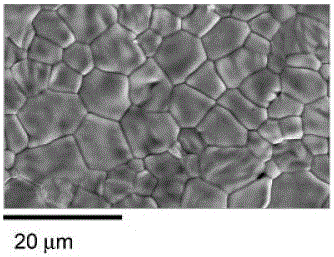
[0235] Press the powder prepared by calcination at 100Mpa and 1000°C with a rated La / W ratio of 5.6 according to the method in Example 1 to prepare a pressed tablet with a diameter of 10mm and a thickness of 1mm; in air, the sample was sintered at 1500°C.
[0236] figure 2 The SEM micrograph of the sample is given, and the particle size is about 10 μm.
Embodiment 3
[0238] The tungstate of the present invention is coated on an alumina carrier by dip coating, and the carrier is dipped in a La and W caprylate solution, and isopropanol and diethanolamine are used in the solution, and drying is performed between each dipping. The membrane is then heat-treated at 800°C, so the catalyst adheres to the membrane surface.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com