Desulfurization and denitrification agent, preparation method and application thereof
A desulfurization and denitrification technology, applied in the field of nanoscale desulfurization and denitration agents, can solve the problems of low utilization rate, complex process, high operating cost, etc., and achieve the effect of increasing specific surface area, improving adsorption function, and low operating cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067]
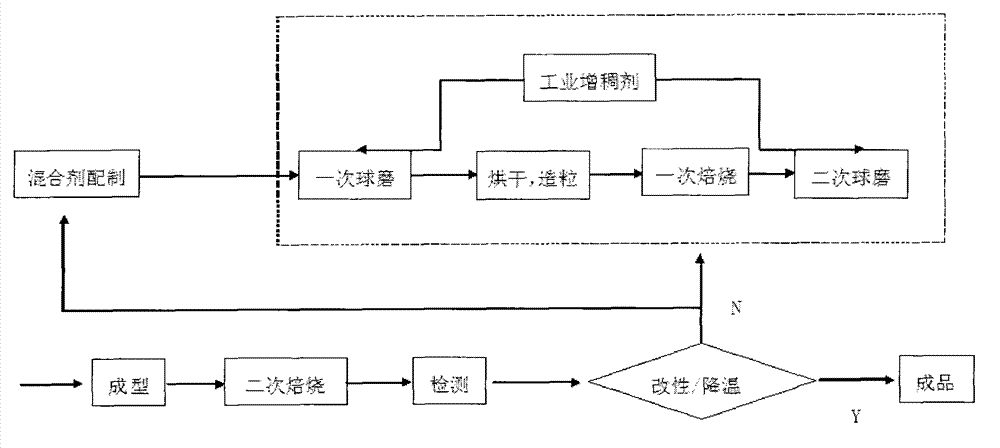
[0068] The present invention also provides a preparation method of desulfurization and denitrification agent, which comprises the following steps:
[0069] (1) Prepare the following components to make a mixture;
[0070] (2) Making the prepared mixture into a nanoscale desulfurization and denitrification agent.
[0071] The components include MgO, SiO 2 , CaO, Fe 2 o 3 、Al 2 o 3 , CuO and MnO 2 ; Optionally, also include KMnO 4 .
[0072] According to the preparation method of the present invention, preferably, comprises the following steps:
[0073] (1) prepare the mixture of the above components in proportion;
[0074] (2) Making the prepared mixture into a nanoscale desulfurization and denitrification agent.
[0075] Among the desulfurization and denitrification agents include:
[0076] MgO is 40~50 parts by weight, preferably 45~48 parts by weight;
[0077] CaO is 0.1 to 1 part by weight, preferably 0.5 to 0.8 parts by weight;
[0078] SiO 2 10 to...
Embodiment 1
[0155] (1) Modulation of desulfurization and denitrification agent:
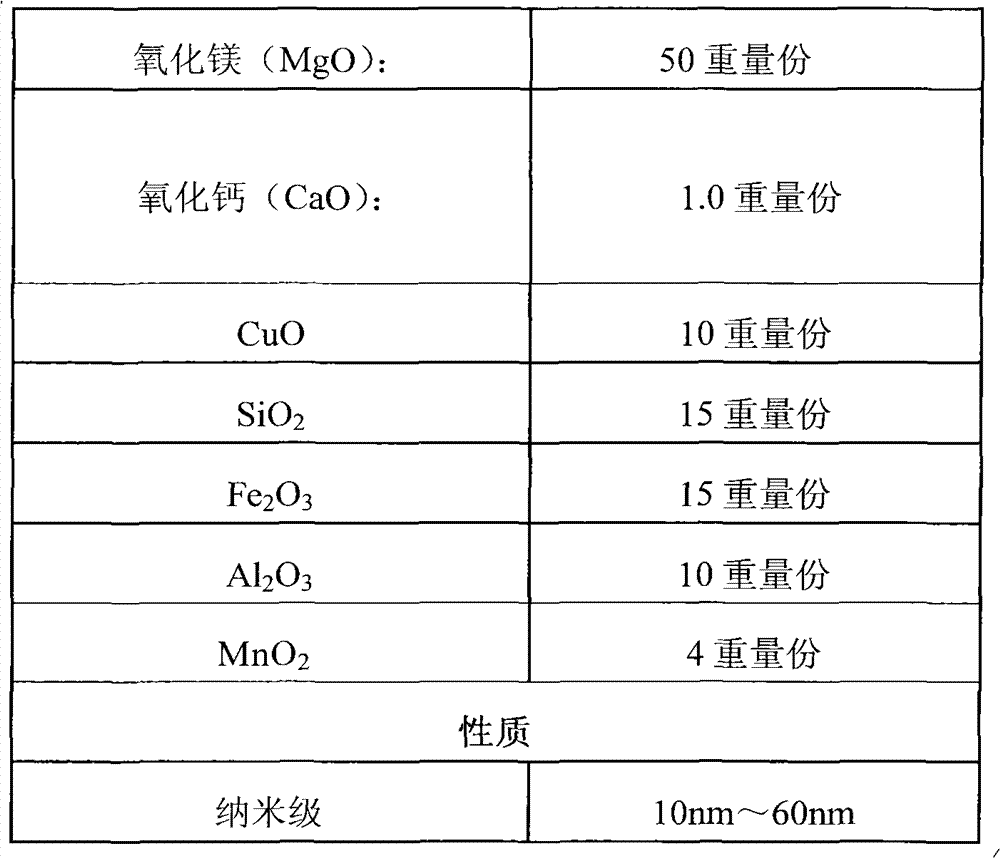
[0156] According to the following formula, mix the components evenly to obtain the desulfurization and denitrification agent.
[0157]
[0158] Put the metal-based oxide desulfurization and denitrification agent prepared according to the ratio in the above table into a high-energy ball mill and grind it into nanoscale specifications within the range of 10nm to 60nm, add water to the desulfurization and denitrification agent nano powder after machine grinding, stir evenly, and then add Enter the industrial thickener HPMC (hydroxypropyl methylcellulose), send the mixed slurry into a single-chamber furnace, and roast it at a temperature range of 600°C to 800°C for 1.5 to 2 hours to obtain a desulfurization and denitrification special Mixture.
[0159] (2) Removal of SO using nanoscale desulfurization and denitrification agents 2 , NO X craft
[0160] 1. Pulping:
[0161] The prepared desulfurization and...
Embodiment 2
[0182] According to the following formula, mix the components evenly to obtain the desulfurization and denitrification agent.
[0183]
[0184]
[0185] Compared with Example 1, Example 2 reduces MgO content by 5 parts by weight, reduces MnO 2 2 parts by weight, increase SiO 2 , Fe 2 o 3 5 parts by weight, increase KmnO 4 2 parts, other conditions are constant, and technological process is also identical with embodiment 1, obtains following parameter list:
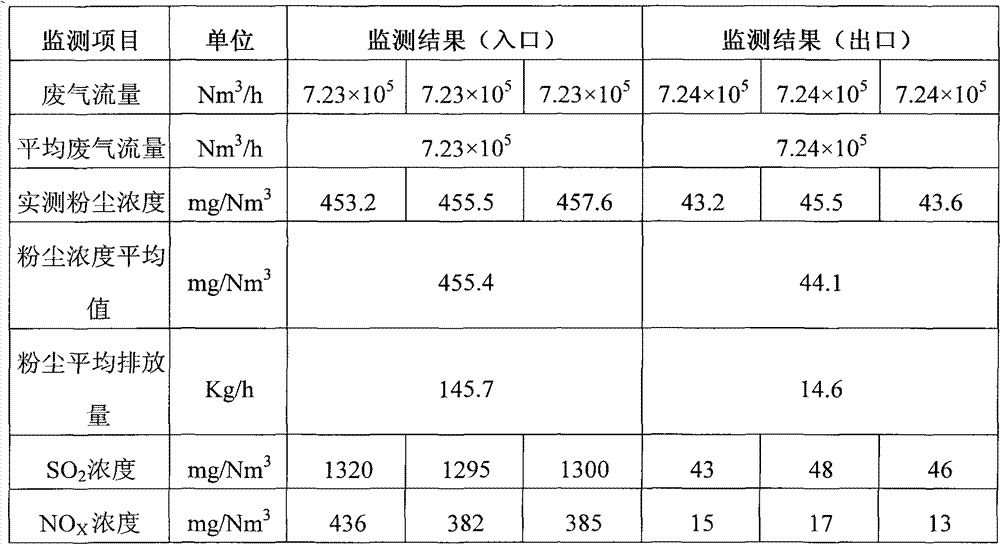
[0186] Table 3: Monitoring results of waste gas emission from sintering machine desulfurization facilities
[0187]
[0188] The desulfurization efficiency reaches 96.9%, and the denitrification efficiency reaches 93.1%.
[0189] At the same time, the consumption parameters of various consumables are as follows:
[0190] Various experimental data
[0191] 1
[0192] The consumption of water, electricity and desulfurization agent is lower than that of the conventional desulfurization system, but ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size range | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com