Method for simultaneously detecting nitrofurans raw drug residue in aquatic product
A technology for nitrofuran and aquatic products, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of increased time and cost, strict detection requirements, quantitative limits, etc., to reduce pretreatment costs, easy operation, quantitative The effect of limit increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. 0.05mol / L NaH 2 PO 4 (pH=3.0) solution configuration method: weigh 7.8g NaH 2 PO 4 2H 2 Add 1000mL of ultrapure water to O, adjust the pH to 3.0 with phosphoric acid, and dilute to 1L.
[0026] 2. Preparation of four mixed standard solutions: Accurately weigh 10 mg each of furaltadone, nitrofurazone, nitrofurantoin, and furazolidone, dilute to 100 mL with methanol, prepare four mixed standard stock solutions of 100 μg / mL, and store at -20 °C. Take 1mL mixed standard stock solution, dilute to 100mL with methanol, and prepare 1μg / mL mixed standard intermediate solution. Pipette mixed standard intermediate solution, use mobile phase (volume ratio: acetonitrile: 0.05M NaH 2 PO 4 =25:75, pH3.0) to prepare standard working solutions with concentrations of 0.010, 0.015, 0.020, 0.025, 0.05, 0.1, and 0.25 μg / mL.
Embodiment 2
[0028] Sample preparation: Grass carp and large yellow croaker were descaled and skinned to take muscle along the back, pulverized by a tissue grinder, stored at -20°C, and thawed at room temperature before measurement.
[0029] Sample pretreatment:
[0030] 1 Extraction: Accurately weigh (5.00±0.05) g of the sample into a 50 mL centrifuge tube, add 8 mL of ethyl acetate, vortex for 1 min, sonicate for 5 min, vortex again for 1 min, centrifuge at 4000 r / min for 5 min, transfer the upper layer to brown In the chicken heart bottle, add 8 mL of ethyl acetate to repeat the extraction, and combine the extracts.
[0031] 2. Concentration: Concentrate the extract to dryness under reduced pressure at 30° C. on a rotary evaporator.
[0032] 3. Purification: add 2mL of n-hexane, vortex for 30s, then add 1mL of mobile phase (volume ratio: acetonitrile:0.05MNaH 2 PO 4 =25:75, pH3.0), mixed for 1min, transferred to a 10mL centrifuge tube, centrifuged at 4000r / min for 5min, took the lowe...
Embodiment 3
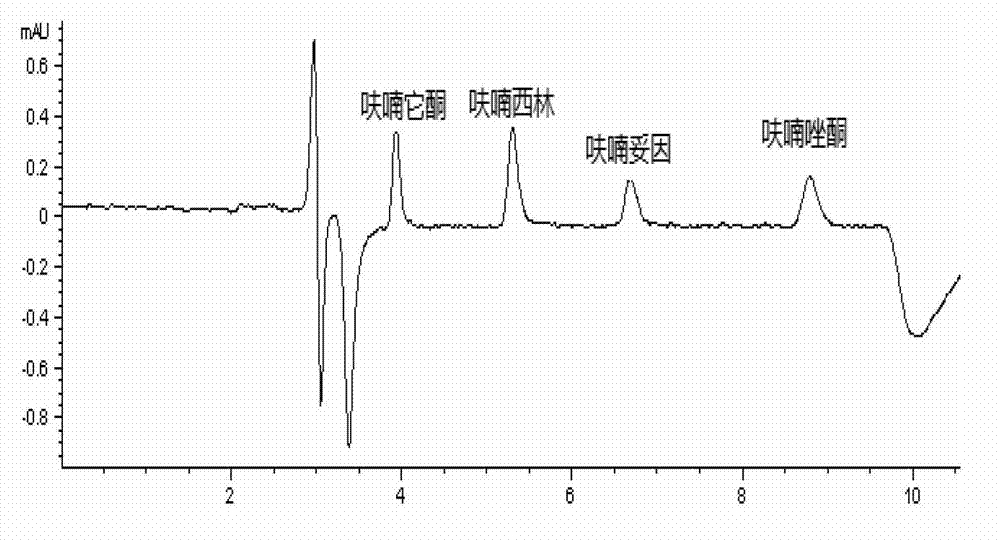
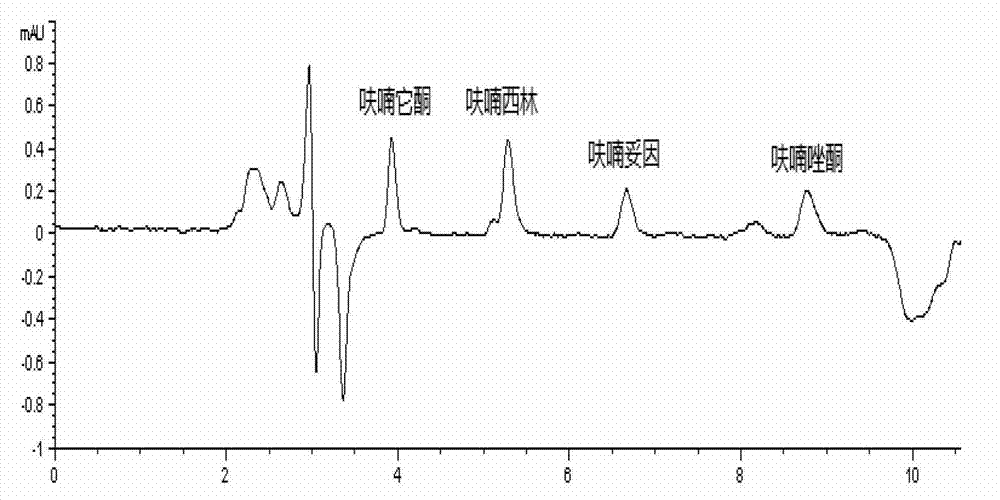
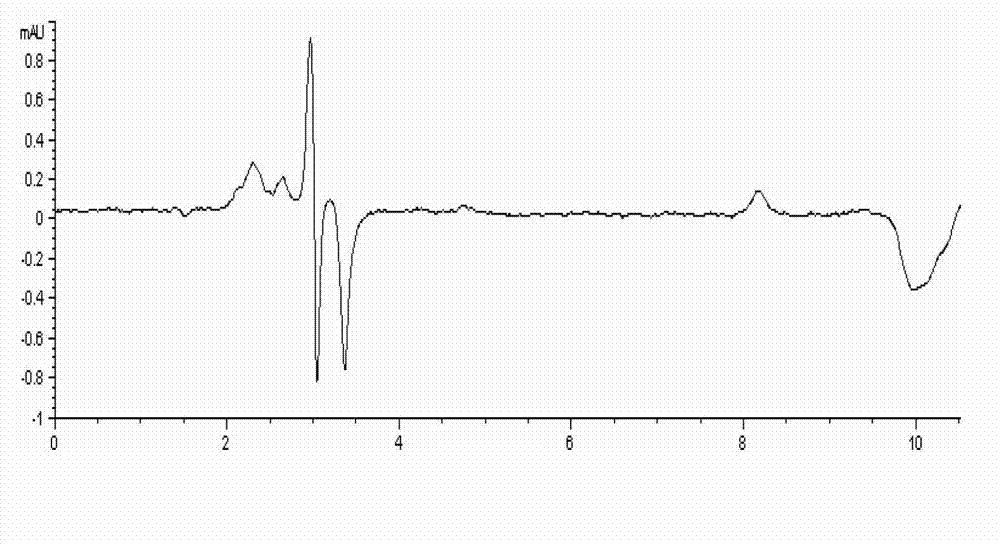
[0037] Chromatographic results: the standard chromatograms of four nitrofuran original drugs are shown in the attached figure 1 . The peak elution times of furaltadone, nitrofurazone, nitrofurantoin and furazolidone were 3.922, 5.294, 6.678 and 8.785 min, respectively.
[0038] Methodology Validation:
[0039] Under the experimental conditions determined by this method, use the mobile phase to prepare a standard working solution with a concentration of 0.010, 0.015, 0.020, 0.025, 0.05, 0.1, and 0.25 μg / mL, and analyze it on the machine. Take the concentration of each drug as the abscissa, The peak area is the ordinate to draw the standard curve. The experimental results show that: in the range of 0.01-0.25 μg / mL, the concentration of the nitrofuran original drug and the peak area have a good linear relationship, and the correlation coefficients are all above 0.998. The four nitrofurans Drug regression equation and correlation coefficient R 2 See Table 2.
[0040] Table 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com