Doxycycline hydrochloride for livestock compound injection and preparation method thereof
A doxycycline hydrochloride injection technology, applied in the field of veterinary drugs, can solve the problems of reducing the solubility, toxicity and tissue irritation of doxycycline hydrochloride, enhancing stability, and prolonging the action time, so as to achieve enhanced long-term effect Antibacterial effect, stable quality, long-term therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
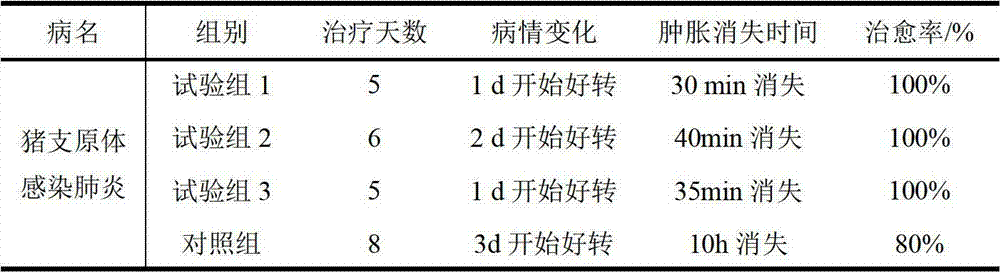
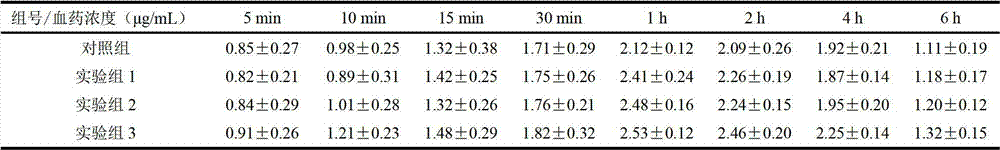
Examples
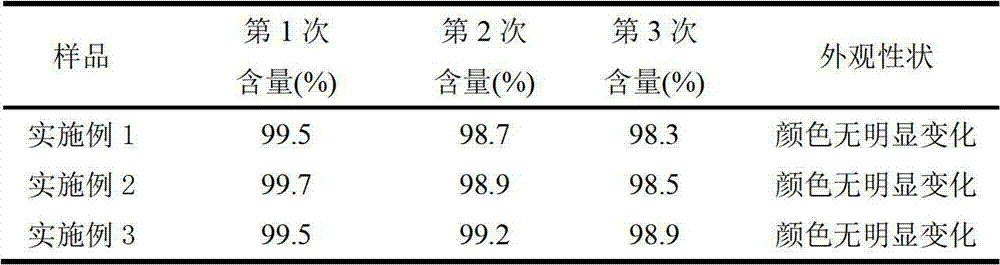
Embodiment 1
[0018] The weight of each component in every 100mL injection is as follows: doxycycline hydrochloride 10g, azithromycin 5g, propylene glycol 20g, α-pyrrolidone 20g, absolute ethanol 10g, PVP K30 2g, magnesium oxide 6g, sodium formaldehyde sulfoxylate 0.3g , ethanolamine 3.2g, and the balance is water for injection.
[0019] The preparation steps are as follows:
[0020] (1) Take a small amount of water for injection, add sodium formaldehyde sulfoxylate, stir until dissolved, then add propylene glycol and α-pyrrolidone in turn, after stirring evenly, add PVP K30 and stir until dissolved;
[0021] (2) Heat the solution prepared in step (1) to 40°C, and keep the temperature at 40°C, add doxycycline hydrochloride and stir evenly, slowly add magnesium oxide, stir while adding until the addition is complete, stir for 10 minutes, Then add ethanolamine to adjust the pH value of the solution, and continue to stir until the solute is completely dissolved;
[0022] (3) Add absolute eth...
Embodiment 2
[0024] The weight of each component in every 100mL injection is as follows: doxycycline hydrochloride 5g, azithromycin 3g, propylene glycol 25g, α-pyrrolidone 15g, absolute ethanol 8g, PVP K30 1g, magnesium oxide 5g, sodium formaldehyde sulfoxylate 0.4g , ethanolamine 3.4g, and the balance is water for injection.
[0025] The preparation steps are as follows:
[0026] (1) Take a small amount of water for injection, add sodium formaldehyde sulfoxylate, stir until dissolved, then add propylene glycol and α-pyrrolidone in turn, after stirring evenly, add PVP K30 and stir until dissolved;
[0027] (2) Heat the solution prepared in step (1) to 40°C, and keep the temperature at 45°C, add doxycycline hydrochloride and stir evenly, slowly add magnesium oxide, stir until the addition is complete, and stir for 10 minutes, Then add ethanolamine to adjust the pH value of the solution, and continue to stir until the solute is completely dissolved;
[0028] (3) Add absolute ethanol to the...
Embodiment 3
[0030] The weight of each component in every 100mL injection is as follows: doxycycline hydrochloride 7g, azithromycin 4g, propylene glycol 30g, α-pyrrolidone 25g, absolute ethanol 9g, PVP K30 1.5g, magnesium oxide 5.5g, sodium formaldehyde sulfoxylate 0.5g, ethanolamine 3.5g, and the balance is water for injection.
[0031] The preparation steps are as follows:
[0032] (1) Take a small amount of water for injection, add sodium formaldehyde sulfoxylate, stir until dissolved, then add propylene glycol and α-pyrrolidone in turn, after stirring evenly, add PVP K30 and stir until dissolved;
[0033] (2) Heat the solution prepared in step (1) to 40°C, and keep the temperature at 45°C, add doxycycline hydrochloride and stir evenly, slowly add magnesium oxide, stir until the addition is complete, and stir for 10 minutes, Then add ethanolamine to adjust the pH value of the solution, and continue to stir until the solute is completely dissolved;
[0034] (3) Add absolute ethanol to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com