Preparation method of magnesium hydrate
A technology of magnesium hydroxide and hydroxide, applied in the direction of magnesium hydroxide, etc., can solve the problems of high production cost, large aggregation tendency, wide distribution, etc., and achieve the effect of low production cost and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
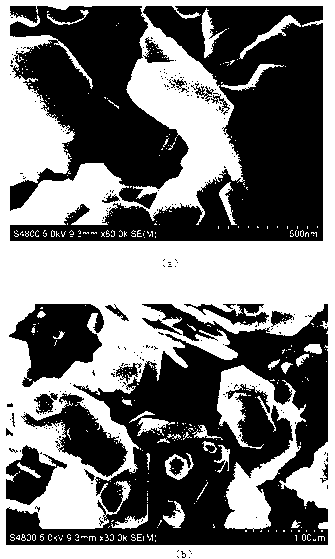
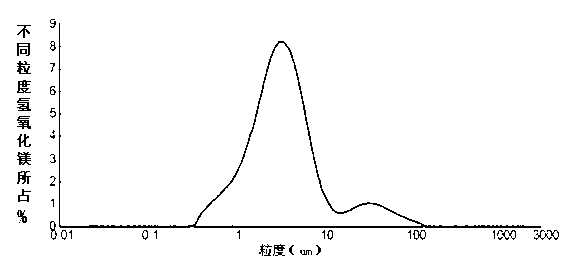
Image
Examples
Embodiment 1
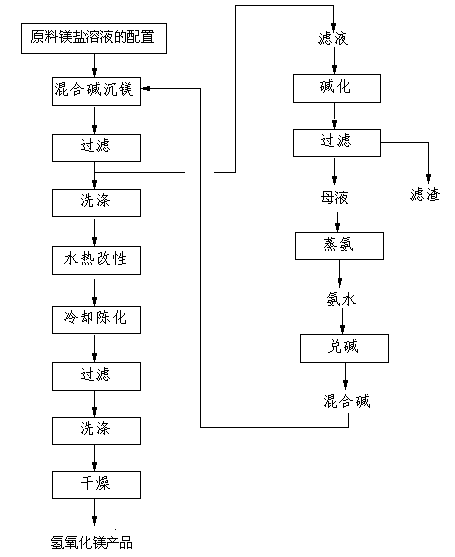
[0041] The first step, the configuration of raw material magnesium salt solution
[0042] The magnesium chloride from the salt mine is mixed with the raw material magnesium salt solution that the molar concentration is 1mol / L;
[0043] The second step is to mix alkali magnesium precipitation
[0044] Take ammonia water with a concentration of 25% by weight and sodium hydroxide from bipolar membrane electrolysis in a ratio of 4:1 by mass to prepare a mixed alkali solution. Drop into the magnesium salt solution prepared in the first step at a rate of 0.1mL / min. When the pH value of the reaction solution system is 10, stop adding the above-mentioned mixed alkali solution dropwise, continue the reaction for half an hour, end the reaction, and generate hydrogen oxide Magnesium precipitation;
[0045] The third step, filter
[0046] The precipitation of the magnesium hydroxide generated in the second step is filtered, and the filtrate obtained by filtering is then processed in th...
Embodiment 2
[0062] The first step, the configuration of raw material magnesium salt solution
[0063] The magnesium chloride separated from the salt mine is mixed with a raw material magnesium salt solution whose molar concentration is 1.5mol / L;
[0064] The second step is to mix alkali magnesium precipitation
[0065] Take ammonia water with a concentration of 25% by weight and potassium hydroxide from bipolar membrane electrolysis in a ratio of 6:1 by mass to prepare a mixed alkali solution. Drop into the magnesium salt solution prepared in the first step at a rate of 0.1mL / min. When the pH value of the reaction solution system is 10, stop adding the above-mentioned mixed alkali solution dropwise, continue the reaction for half an hour, end the reaction, and generate hydrogen oxide Magnesium precipitation;
[0066] The third step, filter
[0067] The precipitation of the magnesium hydroxide generated in the second step is filtered, and the filtrate obtained by filtering is then proce...
Embodiment 3
[0083] The first step, the configuration of raw material magnesium salt solution
[0084] The magnesium sulfate separated from the sea and lake salt is prepared into a raw material magnesium salt solution whose molar concentration is 2mol / L;
[0085] The second step is to mix alkali magnesium precipitation
[0086] Take ammonia water with a concentration of 25% by weight and calcium hydroxide from bipolar membrane electrolysis in a ratio of 9:1 by mass to prepare a mixed alkali solution. Drop into the magnesium salt solution prepared in the first step at a rate of 0.1mL / min. When the pH value of the reaction solution system is 11, stop adding the above-mentioned mixed alkali solution dropwise, continue the reaction for half an hour, end the reaction, and generate hydrogen oxide Magnesium precipitation;
[0087] The third step, filter
[0088] The precipitation of the magnesium hydroxide generated in the second step is filtered, and the filtrate obtained by filtering is then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com