Preparation method of a class of asymmetric spirobifluorene compounds derived from the transformation of functional groups on different fluorene rings
A technology for functional group transformation and spirobifluorene, applied in the field of asymmetric spirobifluorene compounds and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] The preparation of 2,7-bis(malononitrile)-2',7'-bis(triphenylamino)-9,9'-spirobifluorene comprises the following steps:
[0101] (1) Preparation of 4,4'-dimethylbiphenyl
[0102]
[0103] Using cheap and easy-to-obtain 4,4'-dimethylbiphenyl as raw material, under nitrogen protection, weigh 3g (125mmol) of dry magnesium powder, 0.2g (1.2mmol) of ferric chloride in 100ml of dry trichloride in turn. In the necked flask, weigh 11 g (64.3 mmol) of p-bromotoluene into a 50 ml dry constant pressure dropping funnel. Anhydrous 60 ml of tetrahydrofuran was poured into the three-necked flask and vigorously stirred at room temperature. First, 5% p-bromotoluene was added dropwise rapidly, and after the reaction was initiated, it was slowly added dropwise. After the dropwise addition was completed, the reaction was continued for 30 min, the reaction was stopped, cooled to room temperature, and suction filtered to obtain a black filtrate, and the solvent was removed to obtain a b...
Embodiment 2
[0123] Determination of Photoluminescence Spectra of Asymmetric Spirobifluorene Compounds Derived from Functional Group Transformation on Different Fluorene Rings (2,7-bis(malononitrile)-2',7'-bis(triphenylamino)-9 ,9'-spirobifluorene as an example)
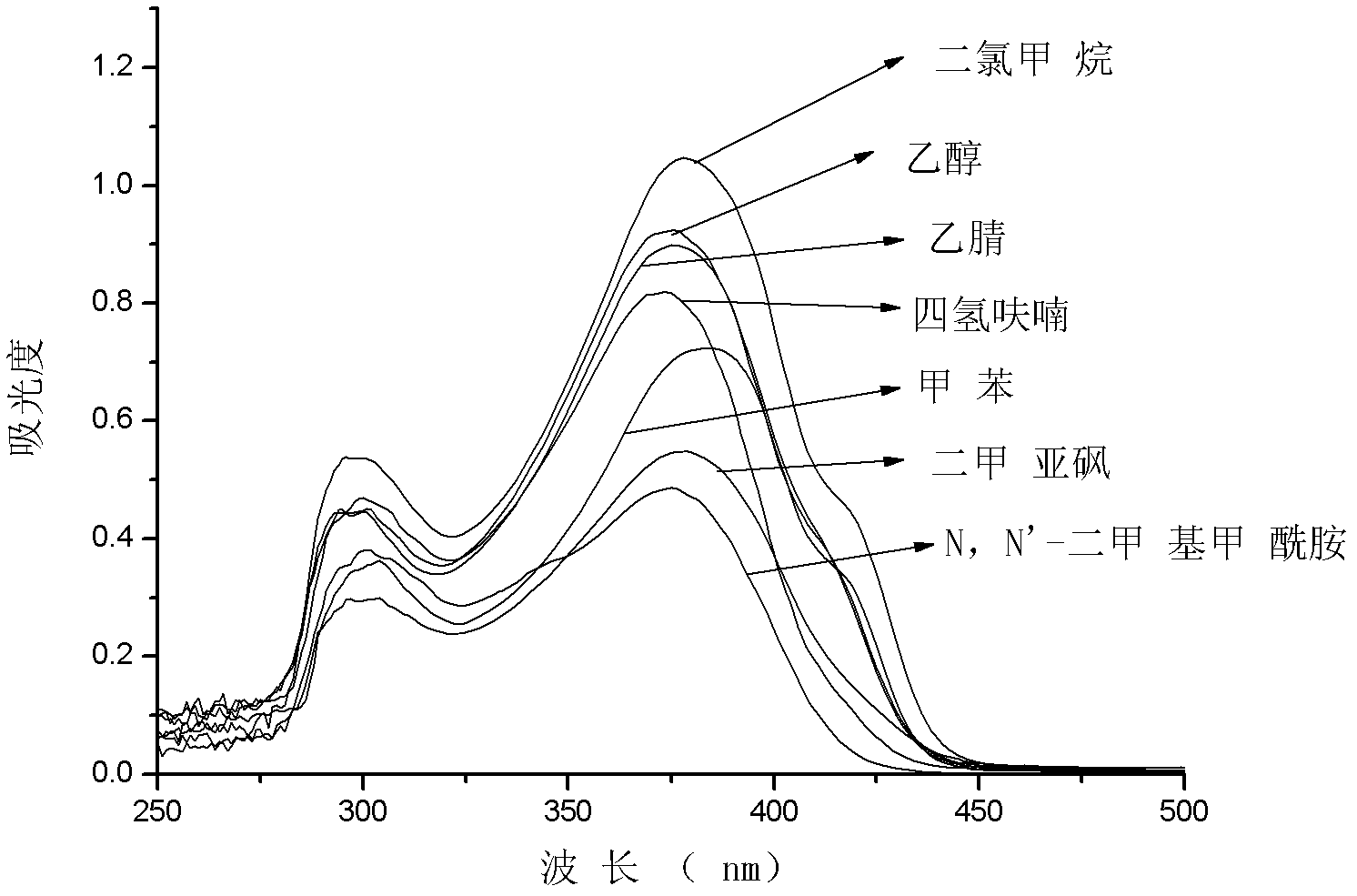
[0124] The product was formulated into 1 × 10 in solvents of different polarities -5 The solution of M was measured by absorption and emission spectra in UV-Vis spectrometer and fluorescence spectrometer, respectively. The UV absorption spectrum is attached figure 2, Fluorescence emission spectrum is attached image 3 . from In Figure 2 It can be clearly seen that the maximum absorption spectrum of the compound is around 380 nm and ends at 440 nm, which fully meets the requirements of the transparency of nonlinear materials. from image 3 It can be clearly seen that the maximum emission spectrum of the compound is around 450 nm, and the maximum emission peak is red-shifted with the increase of solvent polarity. This indi...
Embodiment 3
[0126] Test of second-order nonlinear properties of asymmetric spirobifluorene compounds derived from functional group transformation on different fluorene rings (2,7-bis(malononitrile)-2',7'-bis(triphenylamino)- 9,9'-spirobifluorene as an example)
[0127]Using the lyochromic method, acetonitrile (37.5D) and toluene (2.4D), which have a large difference in dielectric constant, are used as solvents, and the relevant data are measured and entered into the lysochromic equation, and the 2,7-bis(propanediol) is calculated. nitrile)-2',7'-bis(triphenylamino)-9,9'-spirobifluorene β CT μ g is 2344.5. Furthermore, the melting point of 2,7-bis(malononitrile)-2',7'-bis(triphenylamino)-9,9'-spirobifluorene is 177 to 181°C. It can be seen that the "up-down" asymmetric spirobifluorene compound with D-π-A structure has a large second-order nonlinear polarizability, and has very good light transmittance and thermal stability. Solve the current second-order nonlinear material "nonlinearit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com