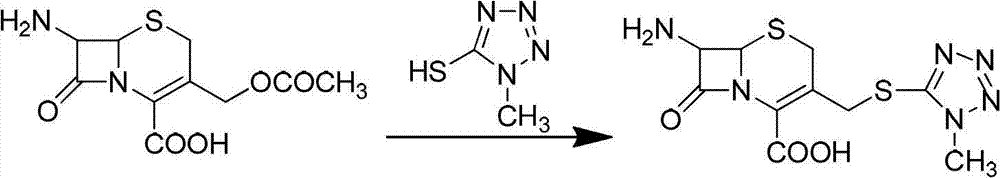
Synthetic method for methoxy-cephalosporin intermediate 7-MAC
A technology of methoxycephalosporin and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of difficult industrialized production and high price, and achieve the effects of simple operation, easy availability of raw materials, and recyclable solvent.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
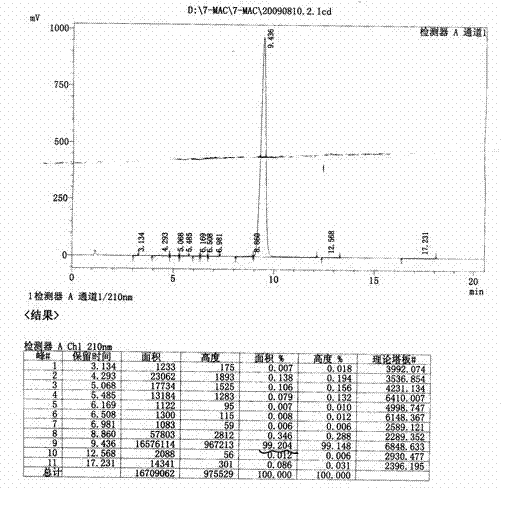
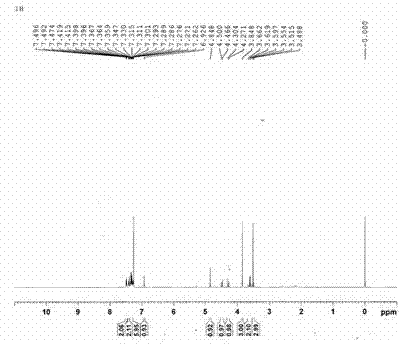
Image
Examples
Embodiment 1
[0056] A kind of synthetic method of metoxyceph intermediate 7-MAC, it is characterized in that comprising the following steps:
[0057] 1. Synthesis of Intermediate I: Add 1000mL of acetonitrile into the reaction bottle, cool to -15~-20℃, slowly add 200mL of concentrated sulfuric acid dropwise, add 128g (1.10mol) of methylmercaptotetrazole, stir for 20min, and divide Add 200g (0.73mol) of 7-ACA in batches, dissolve, then slowly raise the temperature to 40-45°C [reaction temperature in step 1] and react for 2.0-2.5 hours. After the reaction is completed, cool to -12~-15°C, add 40mL of concentrated hydrochloric acid and 50mL of water dropwise, continue to stir for 2.0~3.0h, filter, wash with acetonitrile-acetone mixture, the volume ratio of acetonitrile and acetone is 1:1, every 300 mL each time, and washed 3 times in total. Collect the solid, add 1000 mL of acetone-water mixture, the volume ratio is 3:2, adjust the pH of the solution to 3.6-4.0 with sodium bicarbonate, filter...
Embodiment 2
[0064] The synthesis of intermediate I: increase the thiomethazolium to 152.4g (1.31mmol), and others are the same as in Example 1, and the yield of intermediate I is 92.6%.
[0065] Synthesis of Intermediate II: Add 185g (0.94mol) of benzophenone hydrazone and 180mL of dichloromethane in turn to the reaction flask, stir to dissolve, control the temperature at 25-30°C, add 320g (3.08mol) of industrial grade Electrolytic MnO 2 , Reaction 1.0~2.0h. Filter, and wash the filter residue with dichloromethane (150 mL×3 times). Collect the filtrate and washing liquid, add 180g (0.55mol) of intermediate I, 180mL of dimethyl sulfoxide, stir and raise the temperature to 35-40°C [reaction temperature in step 2] After reacting for 5-8h, lower the temperature below 30°C. Wash with 10% sodium chloride solution (1000mL×2), let stand to separate layers, evaporate the organic layer to dryness at 50-60°C, add 760mL ethyl acetate and 0.6mL triethylamine, stir at room temperature for 12-15h, coo...
Embodiment 3
[0070] Synthesis of Intermediate II: Add 185g (0.94mol) of benzophenone hydrazone and 180mL of dichloromethane in turn to the reaction flask, stir to dissolve, control the temperature at 20-25°C, add 320g (3.08mol) of industrial grade Electrolytic MnO 2 , Reaction 1.0~2.0h. Filter and wash the filter residue with dichloromethane (150mL×3). Collect the filtrate and washing liquid, add 180g (0.55mol) of intermediate I, 180mL of dimethyl sulfoxide, stir and raise the temperature to 50-60°C [reaction temperature in step 2] After reacting for 5-8h, lower the temperature below 30°C. Wash with 10% sodium chloride solution (1000mL×2), let stand to separate layers, evaporate the organic layer to dryness at 50-60°C, add 760mL ethyl acetate and 0.4mL triethylamine, stir at room temperature for 12-15h, cool to 0°C, stirred for 2.0-3.0 h, filtered, washed with ethyl acetate, and dried to obtain Intermediate II as a light red solid with a yield of 90.1% and a liquid phase purity of 98.22%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com