Method for preparing compound sulfachloropyridazine sodium powder
The technology of sulfachlordazine sodium and chlordazine sodium powder is applied in the field of medicines for preventing and treating Pasteurella infection of livestock and poultry, and can solve the problems of no control and prevention of Pasteurella infection of livestock and poultry, and achieves health care prevention, treatment and use safety, Enhanced drug action, diverse and convenient clinical administration methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Formula
[0026] Sulfachlordazine sodium 60g, indomethacin potassium 2g, bentonite 5g, sodium citrate 5g, sodium carboxymethylcellulose 3g, sodium saccharin 2g, soluble starch 23g.
[0027] 2: Preparation method
[0028] The first step: Take bentonite, sodium citrate, and sodium carboxymethylcellulose in the prescribed amount and grind them, pass through an 80-mesh sieve after grinding, and then stir and mix for 15 minutes to obtain A powder without patterns and spots;
[0029] Step 2: Take the prescribed amount of sulfachlordazine sodium, indomethacin potassium and saccharin sodium and pulverize, pass through an 80-mesh sieve after pulverization, and then stir and mix for 15 minutes to obtain B powder without patterns and spots;
[0030] Step 3: Add the above-mentioned B powder to the A powder according to the method of equal addition and stir evenly, then add enough soluble starch and continue to stir and mix for 15 minutes. After checking and confirming that the...
Embodiment 2
[0032] 1. Formula
[0033] Sulfachlordazine sodium 62.5g, indomethacin potassium 3g, bentonite 7.5g, sodium citrate 7.5g, sodium carboxymethylcellulose 4g, sucrose 3g, anhydrous glucose 12.5g.
[0034] 2: Preparation method: the same as the method described in Example 1, only the corrective agent sodium saccharin is replaced with sucrose, and other carrier soluble starch is replaced with anhydrous glucose.
Embodiment 3
[0036] 1. Formula
[0037] Sulfachlordazine sodium 65g, indomethacin potassium 4g, bentonite 10g, sodium citrate 10g, sodium carboxymethylcellulose 5g, sucrose 4g, anhydrous glucose 2g.
[0038] 2: Preparation method: the same as the method described in Example 1, only the corrective agent sodium saccharin is replaced with sucrose, and other carrier soluble starch is replaced with anhydrous glucose.
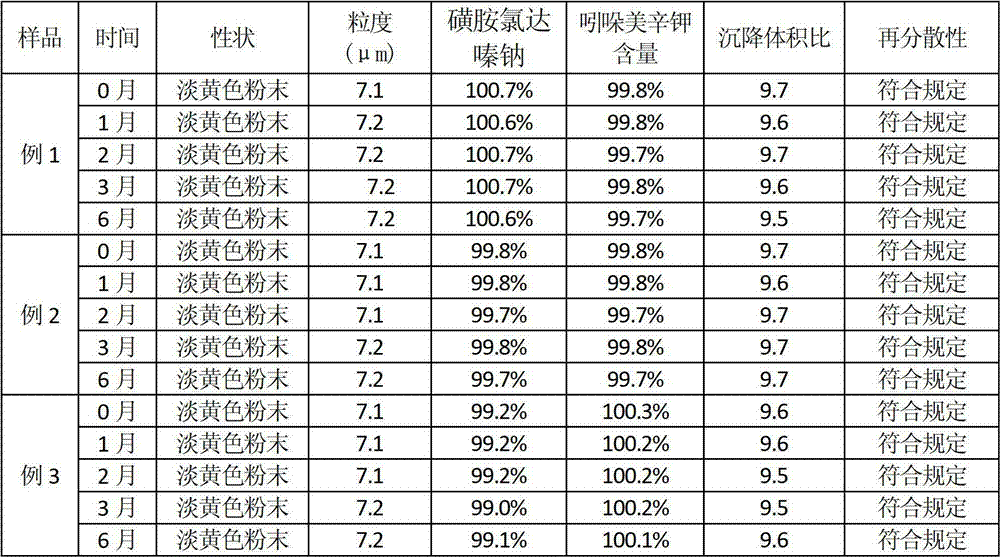
[0039] Stability test of compound sulfachlorperazine sodium powder
[0040] 1. Materials and methods: get the compound sulfachlordazine sodium powder of embodiment 1-3, carry out high-temperature, high-humidity, and strong-light acceleration experiments according to the drug stability test method in the first appendix of "Chinese Veterinary Pharmacopoeia" 2010 edition, and pack according to commercially available , placed in a drug stability test box (Chongqing Yongsheng Experimental Instrument Factory) with a temperature of 40±2°C, a relative humidity of 75±5%, and an illuminat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com