Hemoglobin microcapsule blood substitute and its preparation method
A hemoglobin and microcapsule technology, applied in microcapsules, blood diseases, capsule delivery, etc., can solve problems such as difficulty in obtaining controllable particle size, structure and function, large particle size of microcapsules, and difficulty in oxygen release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Preparation of oxidized heparin (DHP) with a dialdehyde structure
[0046] 0.6 g of heparin was prepared into an aqueous solution with a mass concentration of 1% with distilled water, 0.65 g of sodium periodate was added thereto, and an appropriate amount of ethylene glycol was added to terminate the reaction after 24 hours of dark reaction. After mixing the solution with 0.5g sodium chloride, use 150mL of absolute ethanol to precipitate the product, filter with suction and dissolve the precipitate with deionized water, precipitate with ethanol again, filter with suction, repeat this step 3 times, put the product into a watch glass, 50 ℃ vacuum drying for 24h, you can prepare DHP.
[0047] (2) Biocompatible hemoglobin microcapsules assembled based on Schiffer base bonds
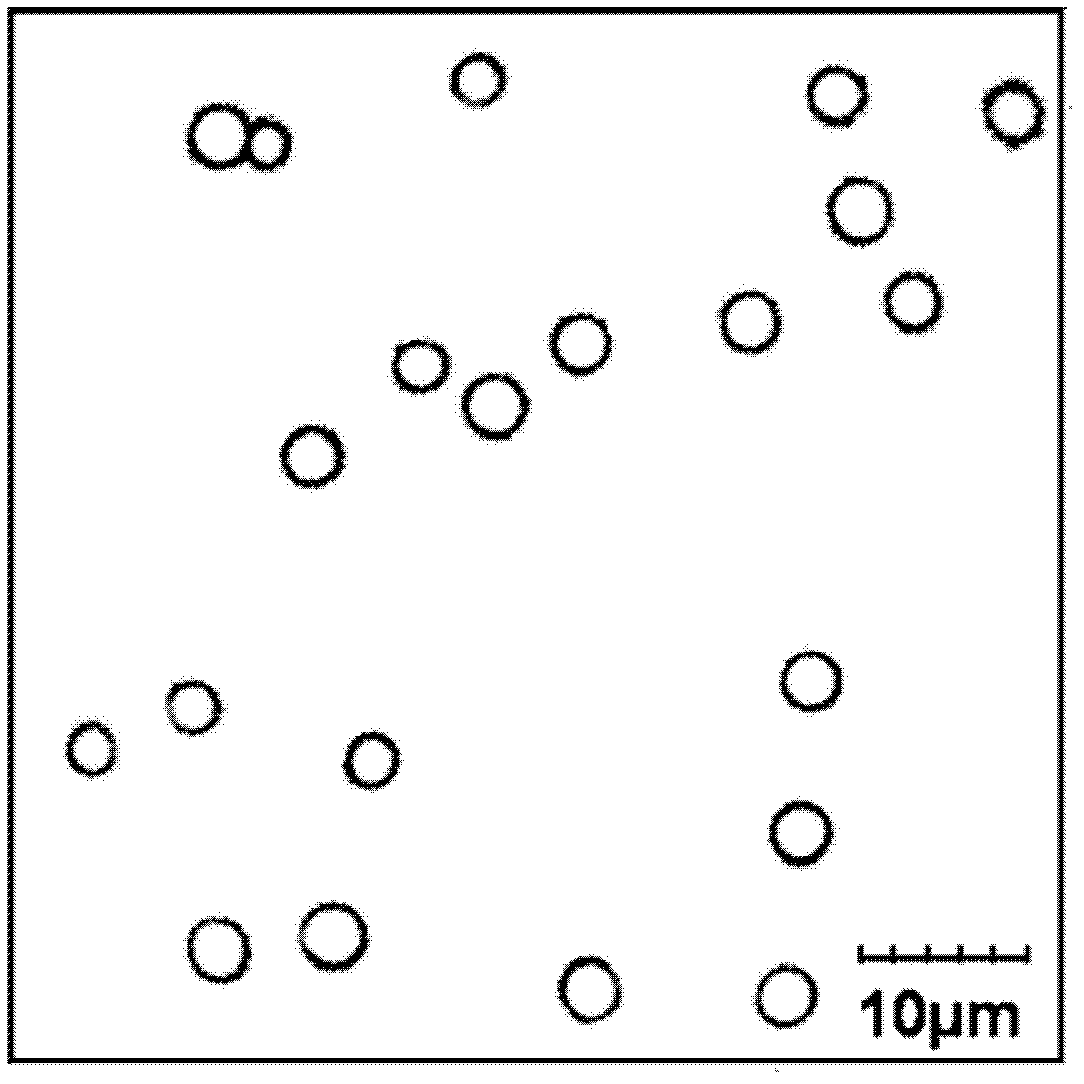
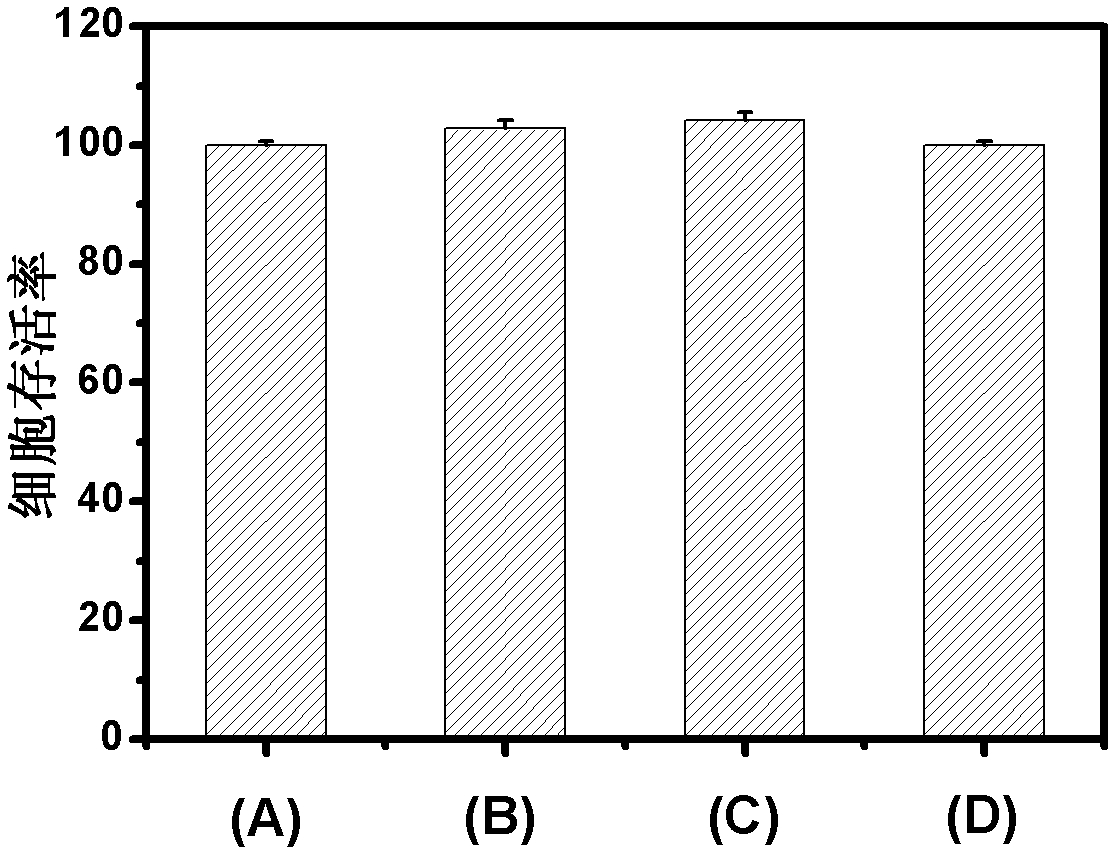
[0048] First, disperse manganese carbonate particles with a particle size of 4 μm into an acetate buffer solution (pH=5) of 4 mg / mL bovine hemoglobin (Hb) containing 0.1 M sodium chloride, shake a...
Embodiment 2
[0056] (1) Preparation of oxidized sodium alginate (alginate dialdehyde, ADA) with a dialdehyde structure
[0057] 4 g of sodium alginate was prepared with distilled water into an aqueous solution with a mass concentration of 2.5%, 4.5 g of sodium periodate was added thereto, and an appropriate amount of ethylene glycol was added to terminate the reaction after 24 hours of dark reaction. After mixing the solution with 10g of sodium chloride, use 200mL of absolute ethanol to precipitate the product, filter with suction and dissolve the precipitate with deionized water, precipitate with ethanol again, filter with suction, repeat this step 3 times, put the product into a watch glass, and keep at 40°C Vacuum drying for 24h, ADA can be prepared.
[0058] (2) Biocompatible hemoglobin microcapsules assembled based on Schiffer base bonds
[0059] First, disperse silica particles with a particle size of 200nm into an acetate buffer solution (pH=5) of 4 mg / mL sheep hemoglobin (Hb) cont...
Embodiment 3
[0061] (1) Preparation of oxidized cellulose (diaaldehyde cellulose, DAC) with a dialdehyde structure
[0062] 3 g of cellulose was prepared into a solution with a mass concentration of 2.5% with distilled water, 3.2 g of sodium periodate was added thereto, and an appropriate amount of ethylene glycol was added to terminate the reaction after 24 hours of dark reaction. After mixing the solution with 8g sodium chloride, use 200mL absolute ethanol to precipitate the product, filter with suction and dissolve the precipitate with deionized water, precipitate with ethanol again, filter with suction, repeat this step 3 times, put the product into a watch glass, and keep at 40°C DAC can be prepared by vacuum drying for 24h.
[0063] (2) Biocompatible microcapsules assembled based on Schiffer base bonds
[0064]First, disperse polystyrene colloidal particles with a particle size of 600nm into an acetate buffer solution (pH=5) of 5 mg / mL bovine hemoglobin (Hb) containing 0.5M sodium c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Cell density | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com