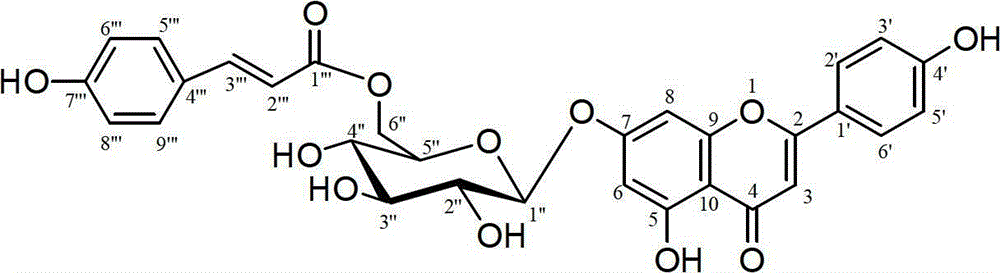
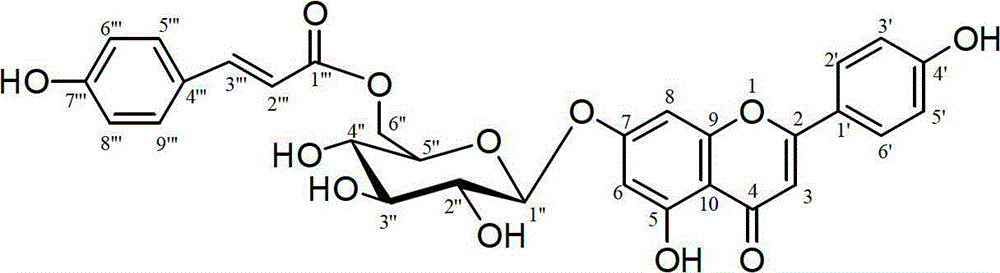
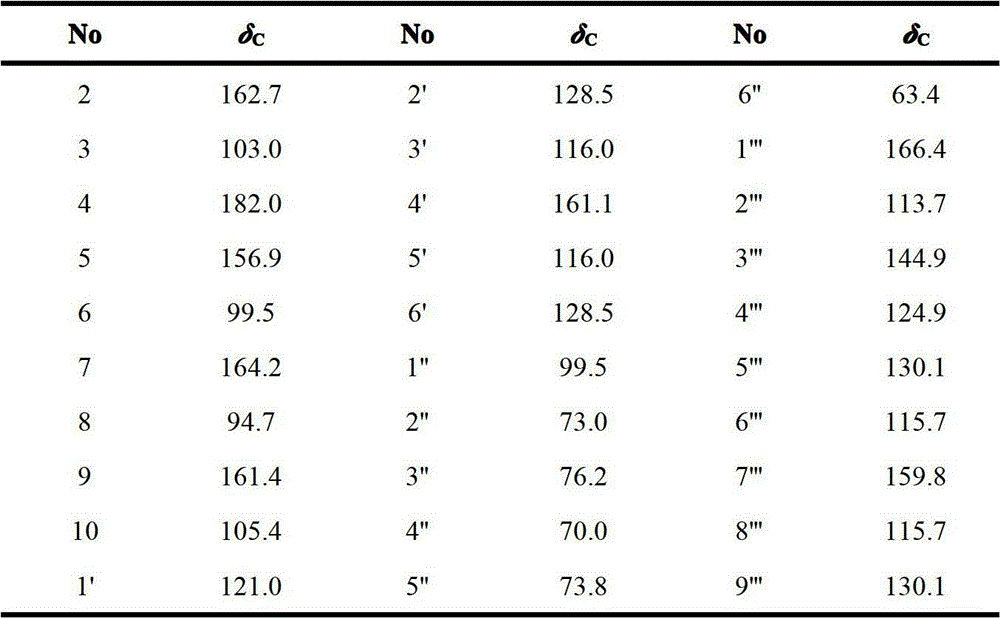
Application of flavonoid glycoside compound on medicine for treating cerebral apoplexy
A technology of flavonoid glycosides and compounds, applied in the field of medicine, can solve problems such as application of unseen compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Extraction and separation of compounds 1
[0016] The whole herb of Clematis clematis collected from Huangnan Tibetan Autonomous Prefecture of Qinghai Province is used as raw material, dried in the shade, after being crushed, 4 kg of coarse powder is taken, and 12 liters of 70% ethanol is added to heat and reflux for extraction, a total of 3 extractions, 2 hours each time . The extracts were filtered and combined, concentrated and dried to obtain a total extract of 985.6 g. The extract was dispersed in 4 liters of water, extracted 3 times with petroleum ether, 4 liters each time, and the extracted water phase was extracted 4 times with n-butanol, 4 liters each time, the n-butanol extract was combined, and the solvent was recovered to obtain 168 grams of n-butanol extraction fraction. The n-butanol extraction part was subjected to silica gel column chromatography (silica gel H for thin-layer chromatography, Qingdao Ocean Chemical Factory), and the chloroform...
Embodiment 2
[0020] Example 2: Extraction and separation of compounds 2
[0021] The whole herb of Clematis clematis collected from Huangnan Tibetan Autonomous Prefecture of Qinghai Province is used as raw material, dried in the shade, after being crushed, 1 kg of coarse powder is taken, and 5 liters of 70% ethanol is added to heat and reflux for extraction, a total of 3 extractions, 4 hours each time . The extracts were filtered and combined, concentrated and dried to obtain 256.8 g of the total extract. The extract was dispersed in 1 liter of water, extracted 3 times with petroleum ether, 1 liter each time, the extracted aqueous phase was extracted 4 times with n-butanol, 1 liter each time, the n-butanol extract was combined, and the solvent was recovered to obtain 46 grams of n-butanol extraction fraction. The n-butanol extraction part was subjected to silica gel column chromatography (silica gel H for thin-layer chromatography, Qingdao Ocean Chemical Factory), and the chloroform-meth...
Embodiment 3
[0028] Example 3: Protective effect of compound APG on cerebral ischemia-reperfusion injury in rats
[0029] Purpose of the test: To observe the effect of the compound APG on the behavioral score and cerebral infarct size of rats with middle cerebral artery occlusion (MCAO), and to evaluate the protective effect of APG on cerebral ischemic injury.
[0030] Experimental animals: 60 male Sprague Dawley (SD) rats, weighing 250-280 g. Free to eat and drink, 25±2℃.
[0031] Reagents: APG, the compound of formula 1 in Example 1, 2,3,5-triphenyltetrazolium chloride (TTC, SigmaAldrich, USA), dimethyl sulfoxide (DMSO, SigmaAldrich, USA).
[0032] animal grouping
[0033] Divide into 6 groups, 8 in each group
[0034] (1) Sham operation group: 0.9% sodium chloride injection
[0035] (2) Model group: 0.9% sodium chloride injection
[0036] (3) Model + DMSO group: 20% DMSO sodium chloride solution
[0037] (4) Low dose group: 25mg / kg
[0038] (5) Medium dose group: 50mg / kg
[0039...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com