Method for producing cyclohexene oxide by reacting cumyl hydroperoxide with cyclohexene
A technology of cumene hydrogen peroxide and epoxy cyclohexane is applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc. Problems such as low hexane selectivity and low catalyst activity, to achieve the effect of reducing the amount, improving the catalytic performance, and increasing the hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
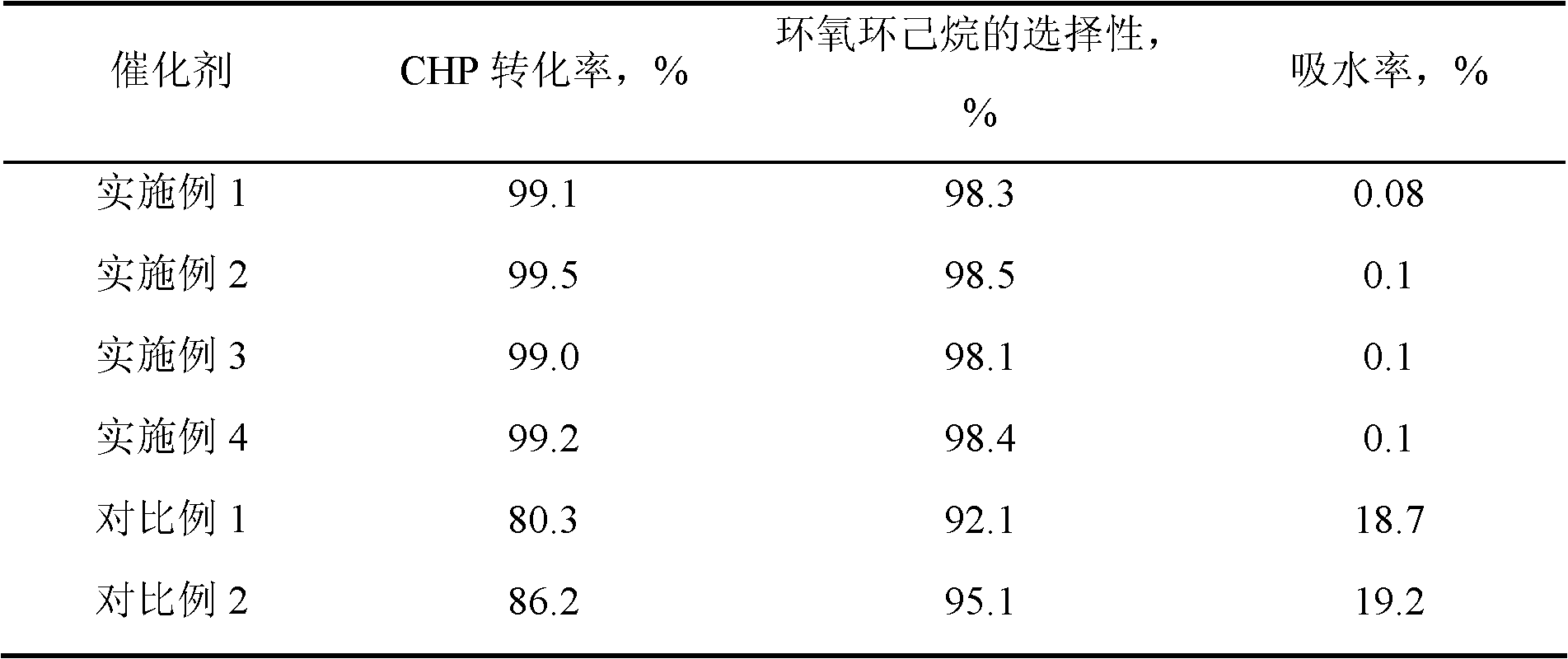
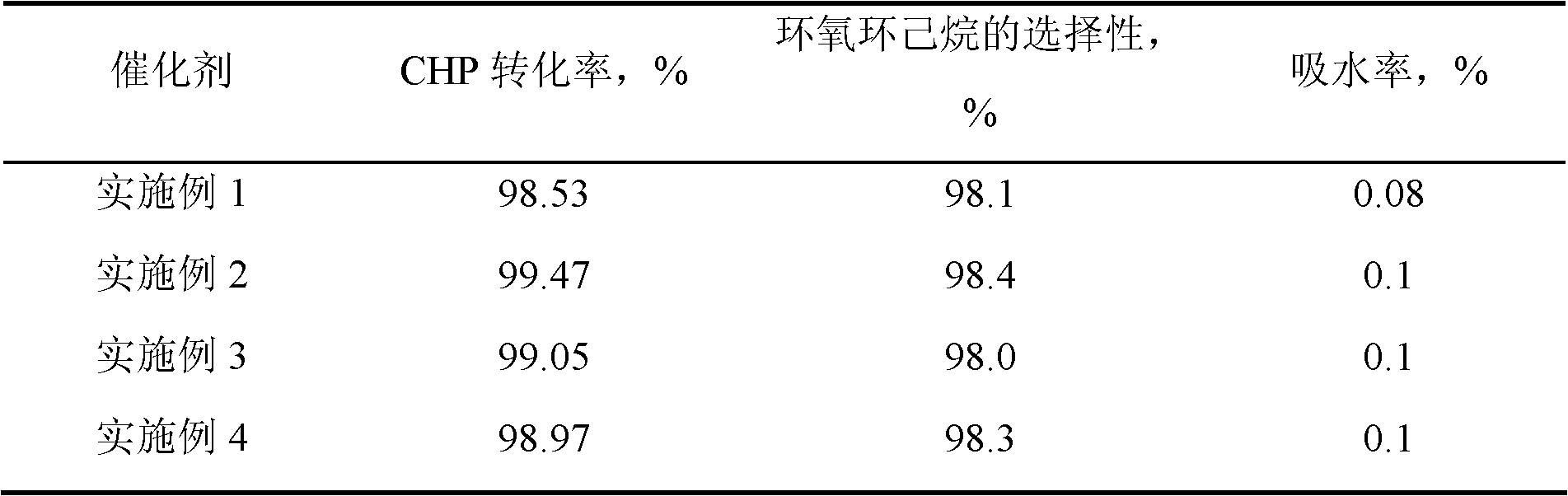
[0015] 7.23 g of n-hexadecylamine was put into a solution consisting of 63 g of deionized water and 32.2 g of ethanol, stirred and dissolved at 50° C. to form solution A. 1 mole of methyltrimethoxysilane and 0.68 g of butyl titanate were dropped into a mixed solution consisting of 6 g of isopropanol and 19 moles of ethyl orthosilicate, and stirred for 30 minutes to form solution B. Pour solution B into solution A, stir for 18 hours, filter, wash and dry, and bake at 350° C. for 8 hours to obtain precursor I. Precursor I was then placed in a quartz tubular reactor at a temperature of 100° C. under a nitrogen atmosphere, passed through N-trimethylsilyl imidazole to react for 1.5 hours, and then purged for 2 hours under a nitrogen atmosphere to obtain HMS structure titanium silicon molecular sieve. Wherein, the molar ratio of each component in the raw material is: methyltrimethoxysilane: ethyl orthosilicate: butyl titanate: hexadecylamine: water = 1: 19: 0.002: 0.03: 3.5, hexame...
Embodiment 2
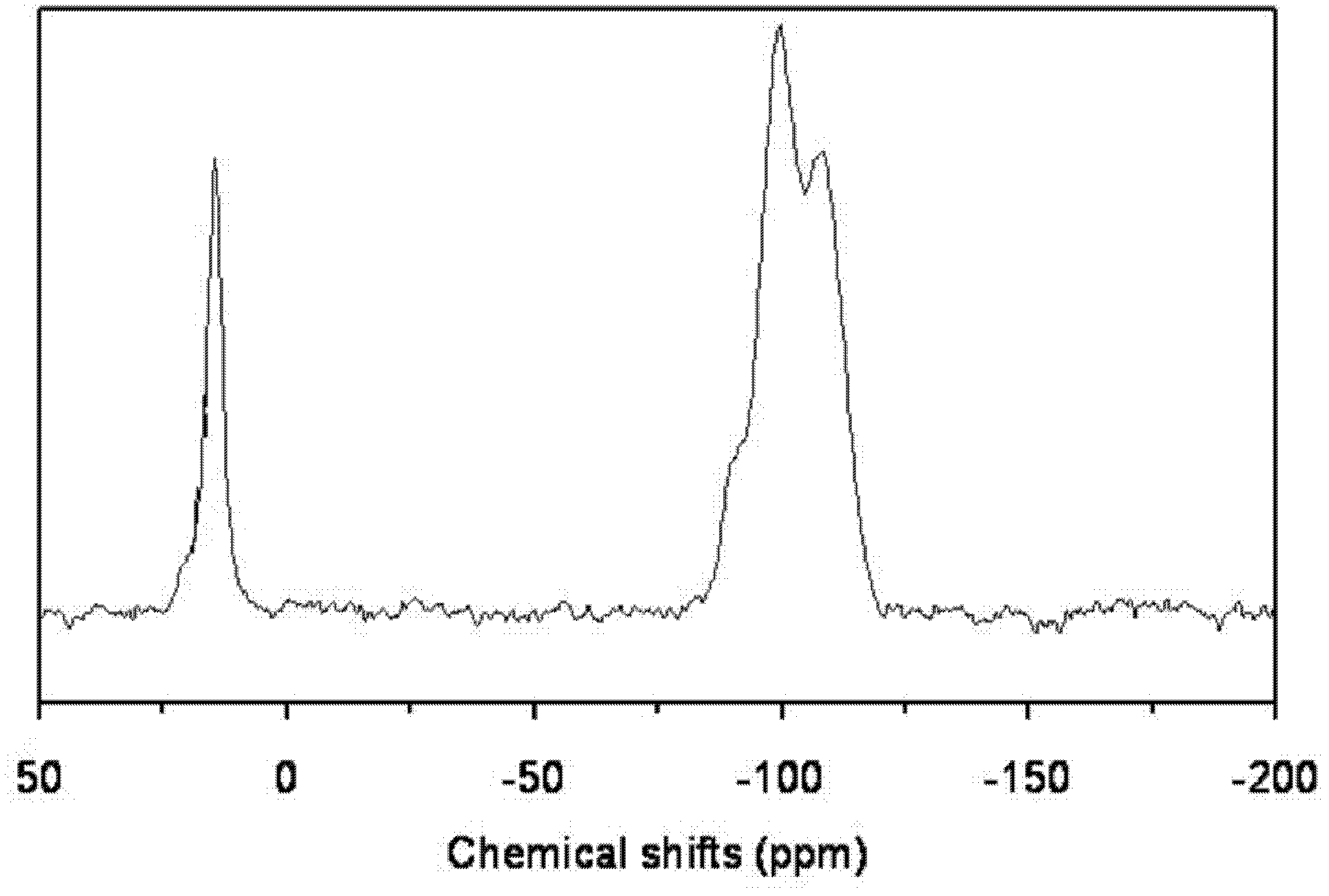
[0017] Same as [Example 1], except that the molar ratio of each component in the raw material is: methyltrimethoxysilane: ethyl orthosilicate: butyl titanate: hexadecylamine: water=1: 19: 0.004: 0.04: 3.5 , under nitrogen atmosphere, the reaction temperature of precursor I and hexamethyldisilazane is 200°C, the reaction time is 3 hours, and the weight ratio of hexamethyldisilazane to precursor I is 0.05. The obtained product29 Si CP / MAS NMR spectrogram is similar to [Example 1].
Embodiment 3
[0019] Same as [Example 1], except that the molar ratio of each component in the raw material is: methyltrimethoxysilane: ethyl orthosilicate: butyl titanate: octadecylamine: water=1: 5: 0.003: 0.04: 4.5 , under a nitrogen atmosphere, the reaction temperature of precursor I and hexamethyldisilazane is 250°C, the reaction time is 5 hours, and the weight ratio of N, O-bistrimethylsilylacetamide to precursor I is 0.005. The obtained product 29 Si CP / MAS NMR spectrogram is similar to [Example 1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com