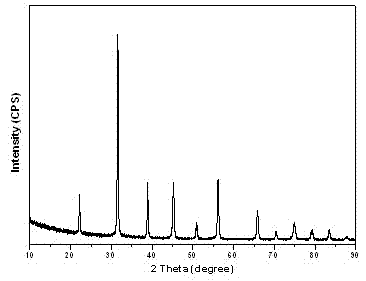
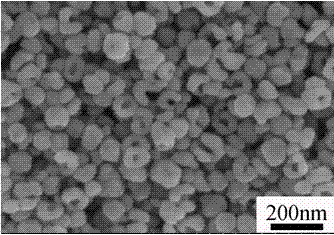
Preparation method of tetragonal-phase barium titanate (BaTiO3) hollow nanocrystal
A technology of phase barium titanate and hollow nanocrystals, which is applied in the field of preparation of tetragonal phase barium titanate hollow nanocrystals, can solve problems such as difficulty in controlling particle size and shape, affecting the electrical properties of electronic components, and coarse powder particles. Achieve the effects of easy control of process conditions, easy scale production, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] 1) Add deionized water, ethylenediamine and hydrochloric acid to tetrabutyl titanate, the molar ratio of tetrabutyl titanate, deionized water, ethylenediamine and hydrochloric acid is 1:260:0.3:0.03 under normal pressure and Stir at 80° C. for 6 hours, control the pH value of the reactant to 2, and obtain a titanium-containing water-soluble sol.
[0022] 2) Measure and weigh barium acetate according to the molar ratio of barium acetate and tetrabutyl titanate of 3:1, add barium acetate into deionized water, and stir well to form an aqueous solution of barium acetate;
[0023] 3) Dissolve potassium hydroxide in deionized water, add the aqueous solution of barium acetate prepared in step 2) to the aqueous potassium hydroxide solution, and then add it to the titanium-containing water-soluble sol in step 1), to obtain a solution containing barium and titanium Suspension of oxyhydroxide precipitates;
[0024] 4) Transfer the precipitated suspension containing barium and tit...
example 2
[0027] 1) Add deionized water, acetylacetone and nitric acid to tetrabutyl titanate, the molar ratio of tetrabutyl titanate, deionized water, acetylacetone and nitric acid is 1:280:0.37:0.04. Stir at normal pressure and 60° C. for 10 hours, control the pH value of the reactant to 3, and obtain a titanium-containing water-soluble sol.
[0028] 2) Measure and weigh barium acetate according to the molar ratio of barium acetate and tetrabutyl titanate of 3:1, add barium acetate into deionized water, and stir well to form an aqueous solution of barium acetate;
[0029] 3) Dissolve potassium hydroxide in deionized water, add the aqueous solution of barium acetate prepared in step 2) to the aqueous potassium hydroxide solution, and then add it to the titanium-containing water-soluble sol in step 1), to obtain a solution containing barium and titanium Suspension of oxyhydroxide precipitates;
[0030] 4) Transfer the obtained suspension containing precipitated oxyhydroxides containing...
example 3
[0033] 1) Add deionized water, acetylacetone and hydrochloric acid to tetrabutyl titanate, the molar ratio of tetrabutyl titanate, deionized water, acetylacetone and hydrochloric acid is 1:210:0.45:0.01. Stir at normal pressure and 50° C. for 24 hours, control the pH value of the reactant to 3, and obtain a titanium-containing water-soluble sol.
[0034] 2) Measure and weigh barium acetate according to the molar ratio of barium acetate and tetrabutyl titanate of 3:1, add barium acetate into deionized water, and stir well to form an aqueous solution of barium acetate;
[0035] 3) Dissolve potassium hydroxide in deionized water, add the aqueous solution of barium acetate prepared in step 2) to the aqueous potassium hydroxide solution, and then add it to the titanium-containing water-soluble sol in step 1), to obtain a solution containing barium and titanium Suspension of oxyhydroxide precipitates;
[0036] 4) Transfer the obtained suspension containing precipitated oxyhydroxide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com