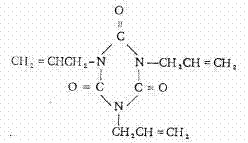
Preparation method of triallyl isocyanurate
A technology of triallyl isocyanurate and sodium isocyanurate, applied in the direction of organic chemistry and the like, can solve problems such as being unsuitable for industrial production, and achieve the effects of high product yield, good product quality and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 195g of sodium isocyanurate and 600g of acetonitrile into a four-necked flask with a condenser. At the same time add 2g of cuprous chloride and 8g of triethylamine. The temperature is raised to 70-75℃, and 345g of chloropropene is slowly added dropwise. The time is 3.5-4 hours. After the chloropropene is added dropwise, the temperature is maintained at 80-85°C for 4 hours. After the reaction, the solid phase was filtered off with suction. After recovering all the solvents in the liquid phase, 205g of a colorless and transparent liquid triallyl isocyanurate product was distilled out at 113~115℃ / 133.3Pa. The yield was 82.16% (in isocyanate As sodium urate), the content is 98. 12% (HPLC).
Embodiment 2
[0024] Add 195g of sodium isocyanurate and 500g of N,N-dimethylformamide into a four-necked flask with a condenser. At the same time, add 2g of cuprous chloride and 8g of tributylamine. The temperature is increased to 80-85°C, and it starts to drip slowly. Add 345 g of chloropropene for 3 to 3.5 hours, and keep the temperature at 90-95°C for 3 hours after the chloropropene is added. After the reaction, the solid phase was filtered off with suction, and all the solvents were recovered under reduced pressure in the liquid phase. After distilling out 215 g of a colorless and transparent liquid triallyl isocyanurate product at 113~115℃ / 133.3Pa, the yield was 86.17%. As sodium isocyanurate), the content is 98.46% (HPLC).
Embodiment 3
[0026] Add 195g of sodium isocyanurate and 500g of dimethyl sulfoxide into a four-necked flask with a condenser. At the same time, add 2g of copper powder and 8g of triethylamine. The temperature is increased to 90-95℃, and 345g of chloropropene is slowly added dropwise. The addition time is 3-3.5 hours. After the chloropropene is added dropwise, the temperature is maintained at 100-105°C to react for 3 hours. After the reaction, the solid phase was filtered off with suction, and all the solvents were recovered in the liquid phase under reduced pressure. After distilling out 212g of a colorless and transparent liquid triallyl isocyanurate product at 113~115℃ / 133.3Pa, the yield was 84.97% (with As sodium isocyanurate), the content is 98. 71% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com