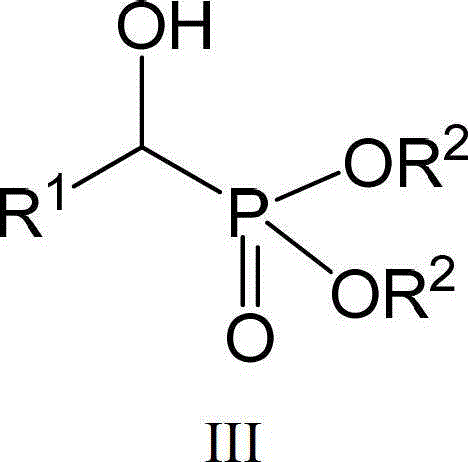
A kind of double metallocene based rare earth guanidinium compound and its preparation method and the preparation method of hydroxy phosphite compound
A technology of hydroxyphosphite and compounds, which is applied in the field of rare earth metal catalyst preparation, can solve the problems that have not been reported yet, and the research scope is narrow.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
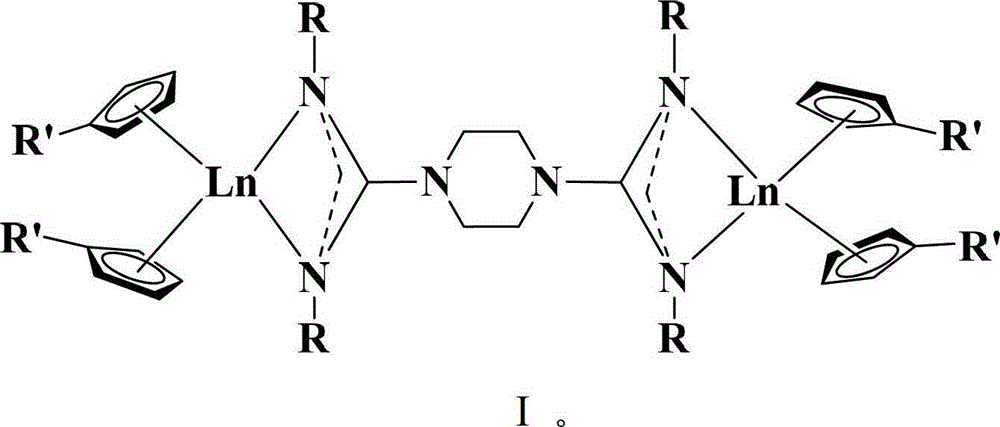
[0035] The technical scheme adopted in the present invention is: a kind of preparation method of double metallocene-based rare earth guanidine complex, comprising the following steps:
[0036] a) Under anhydrous and oxygen-free conditions, in an inert atmosphere, the bridged biguanide and n-butyllithium were reacted in a solvent at 0°C for 2 hours at a molar ratio of 1:2 to obtain a bridged biguanide lithium salt;
[0037] b) Add (R'C to the bridged biguanide lithium salt obtained in step a) 5 h 4 ) 2 Solution of LnCl, continue to react for 12 hours, the reaction temperature is 10-90°C; and the boiling point of the solvent is not exceeded; wherein Ln is ytterbium, erbium, yttrium or samarium;
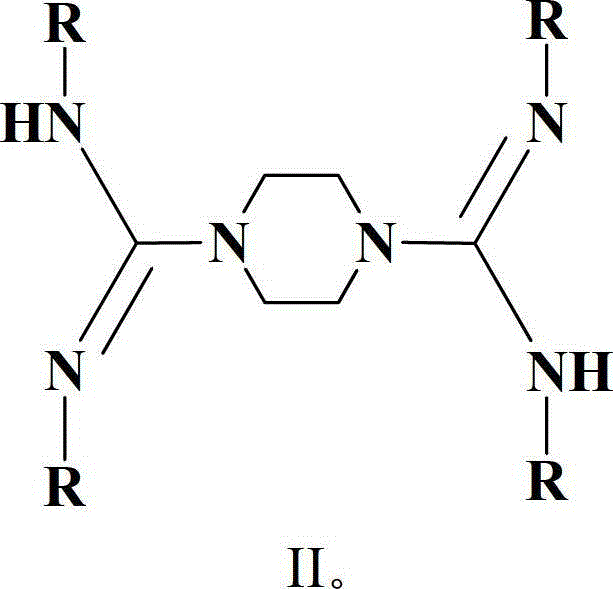
[0038] Wherein, the general formula of the bridged biguanide is {[H(RN) 2 CN(CH 2 ) 2 ]} 2 And have the structure shown in formula II, wherein R is isopropyl or cyclohexyl; Its synthetic method can refer to literature: Zhang, W.X.; Nishiura, M.; Hou, Z.M.Chem.Eur.J.2007,13,4037, ...
Embodiment 1
[0063] prepare {(C 5 h 5 ) 2 Yb[( i PrN) 2 CN(CH 2 ) 2 ]} 2 :
[0064] At 0°C, slowly add 3.94 mmoles of n-butyllithium hexane solution dropwise to a tetrahydrofuran solution (1.97 mmoles) containing piperazinyl-bridged biguanide, react for 2 hours, then add (C 5 h 5 ) 2 YbCl (3.94 mmol), reacted overnight at 25°C, removed THF, added toluene for extraction, centrifuged to remove lithium chloride, concentrated, and stood at room temperature, 1.56 g (1.65 mmol) of brick red crystals were precipitated, with a yield of 84%. Decomposition temperature: 130-132°C. Elemental analysis: C, 48.47; H, 5.92; N, 9.03; Yb, 36.82. Infrared absorption spectrum data: 2966s, 2927w, 2854w, 1624s, 1458m, 1385s, 1362s, 1257s, 1165s, 1134m, 1065w, 1007m, 933m, 852w, 733w, 548w. The above data prove that the compound was successfully prepared.
Embodiment 2
[0066] prepare {(C 5 h 5 ) 2 Er[( i PrN) 2 CN(CH 2 ) 2 ]} 2 :
[0067] At 0°C, slowly add 3.94 mmoles of n-butyllithium hexane solution dropwise to a tetrahydrofuran solution (1.97 mmoles) containing piperazinyl-bridged biguanide, react for 2 hours, then add (C 5 h 5 ) 2ErCl (3.94 mmol), reacted overnight at 25°C, removed THF, added toluene for extraction, centrifuged to remove lithium chloride, concentrated, and stood at room temperature, 1.50 g (1.62 mmol) of pink crystals were precipitated, with a yield of 82%. Decomposition temperature: 138-140°C. Elemental analysis: C, 48.48; H, 5.97; N, 8.91; Er, 35.85. Infrared absorption spectrum data: 2966s, 2927w, 2858w, 1624s, 1458m, 1389s, 1362s, 1257s, 1165s, 1134m, 1061w, 1003m, 933m, 841w, 737w, 555w. The above data prove that the compound was successfully prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com