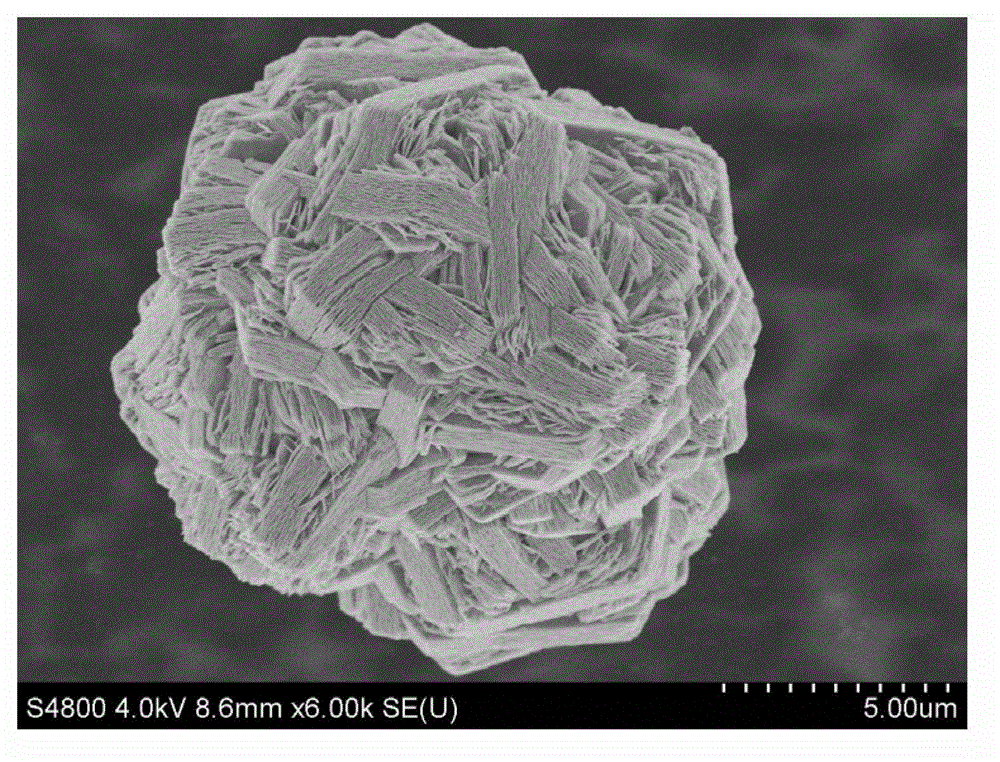
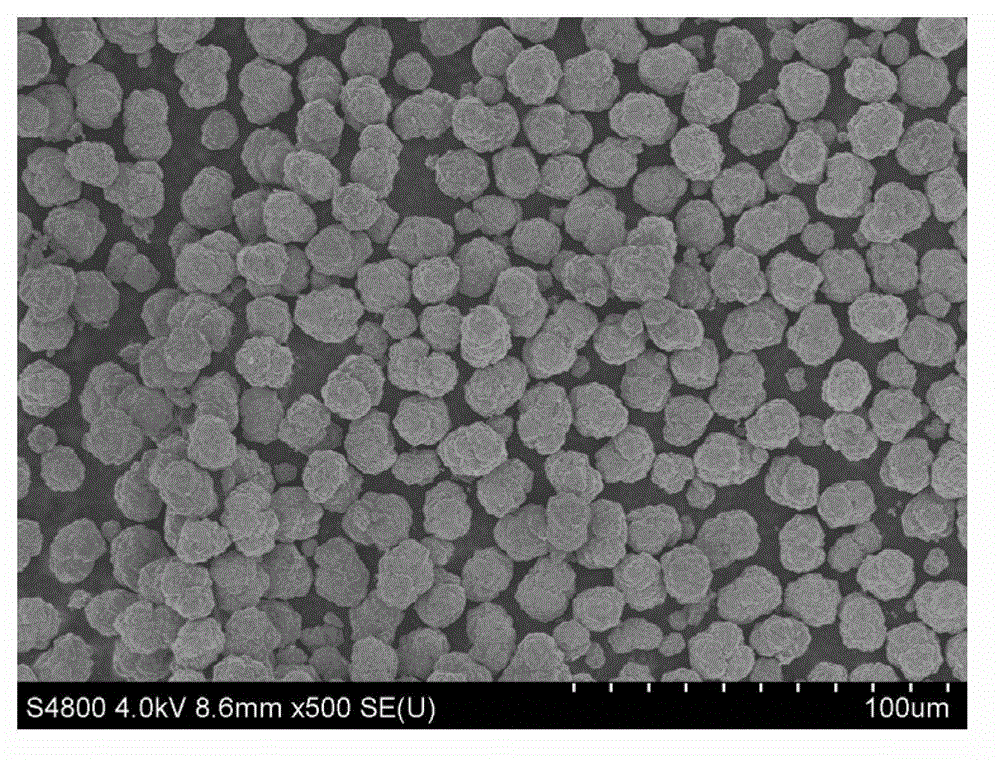
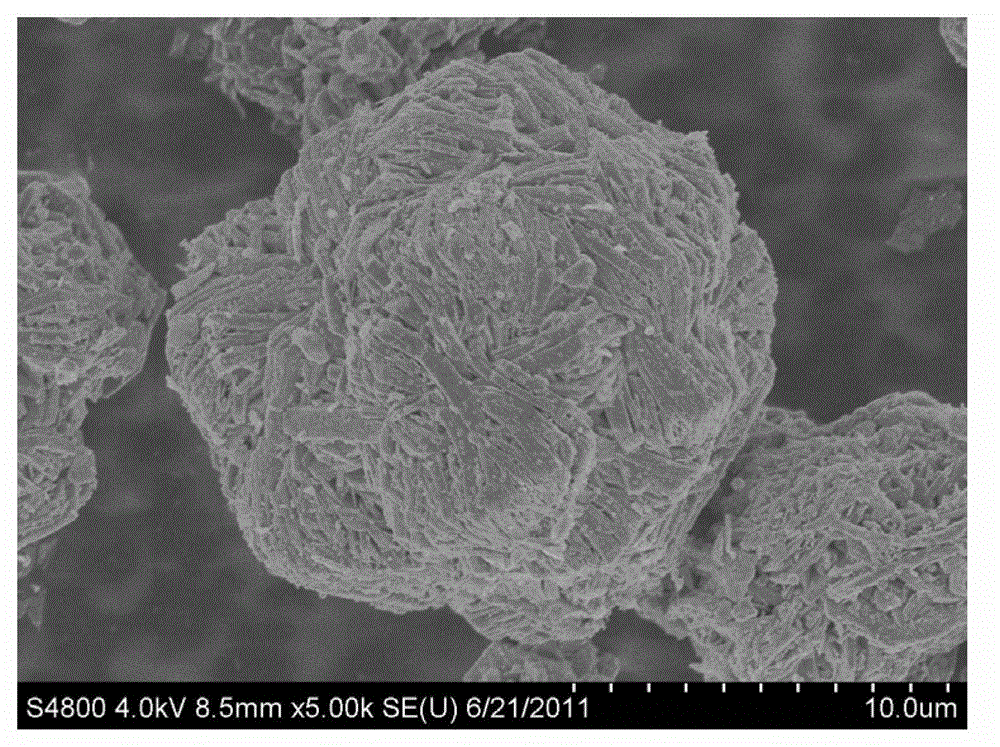
Nickel cobalt manganese hydroxide precursor and preparation method thereof
A nickel-cobalt-manganese-hydroxide, hydroxide technology, applied in electrical components, battery electrodes, circuits, etc., can solve problems such as unfavorable lithium ion diffusion, and achieve the effects of good spherical shape, good particle fluidity, and uniform particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] In order to obtain the nickel-cobalt-manganese hydroxide precursor with the above-mentioned morphology, the present invention also discloses a preparation method of the above-mentioned nickel-cobalt-manganese hydroxide precursor, comprising the following steps:
[0033] Mix nickel salt, manganese salt and cobalt salt to obtain a mixed solution, the total concentration of nickel salt, manganese salt and cobalt salt in the mixed solution is 1 ~ 2mol / L, nickel ion in the nickel salt, cobalt in the cobalt salt The molar ratio of ions to manganese ions in the manganese salt is x:y:1-x-y, where 0<x<1, 0<y<1, x+y<1;
[0034] Mixing the additive with the mixed solution to obtain a first mixed solution, the ratio of the moles of the additive to the total moles of the nickel ions, cobalt ions and manganese ions is 0.005 to 0.1:1;
[0035] The first mixed solution, the sodium hydroxide solution with a concentration of 2 to 10 mol / L and the ammonia solution with a concentration of ...
Embodiment 1
[0052] (1) Prepare a mixed solution of nickel sulfate, cobalt sulfate and manganese sulfate, the molar ratio of nickel sulfate, cobalt sulfate and manganese sulfate is 1 / 3:1 / 3:1 / 3, nickel sulfate, cobalt sulfate in the mixed solution The total concentration with manganese sulfate is 2mol / L;
[0053] (2) In the mixed solution, add 2% triethanolamine of the total moles of nickel sulfate, cobalt sulfate and manganese sulfate;
[0054] (3) Prepare a sodium hydroxide precipitant solution with a concentration of 5mol / L;
[0055] (4) Prepare an ammonia solution with a concentration of 5mol / L;
[0056] (5) Add the above three solutions continuously into the reactor with stirring, and control the amount of ammonia added to be NH 3 h 2 O: (Ni+Co+Mn)=1:1; adjust the flow rate of the sodium hydroxide precipitant solution to control the pH value of 11, pass argon protection during the reaction process, and age for 36 hours after the reaction for 20 hours. The reaction process is consis...
Embodiment 2
[0059] (1) Prepare a mixed solution of nickel chloride, cobalt chloride and manganese chloride, the molar ratio of nickel chloride, cobalt chloride and manganese chloride is 1 / 3:1 / 3:1 / 3, the mixed solution The total concentration of nickel chloride, cobalt chloride and manganese chloride in the medium is 2mol / L;
[0060] (2) In the mixed solution, add nickel chloride, cobalt chloride and polymaleic anhydride of 5% of the total moles of manganese chloride;
[0061] (3) Prepare a sodium hydroxide precipitant solution with a concentration of 10mol / L;
[0062] (4) Prepare an ammonia solution with a concentration of 8mol / L;
[0063] (5) Add the above three solutions continuously into the reactor with stirring, and control the amount of ammonia added to be NH 3 h 2 O: (Ni+Co+Mn)=8:1; adjust the flow rate of the sodium hydroxide precipitant solution to control the pH value to 10, pass nitrogen protection during the reaction process, and age for 48 hours after 20 hours of reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com