Oxazole derivatives useful as modulators of FAAH
A compound and alkyl technology, applied in anti-inflammatory agents, drug combinations, non-central analgesics, etc., can solve problems such as weight, calorie intake, and liver triglyceride levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0409] Preparation of lysates and microsomes
[0410] CHO cells expressing FAAH were used to prepare crude cell lysates or microsomal fractions. To harvest the cells, decant the tissue culture medium slowly and replace the monolayer with Ca-free ++ Mg ++ The cells were washed three times with PBS and recovered after 15 min in enzyme-free dissociation medium (Millipore Corp, Billerica, MA). Cells were harvested by centrifugation at 2000 rpm for 15 min, and the cell pellet was washed with 50 mM HEPES (pH 7.4) containing 1 mM EDTA and the protease inhibitors aprotinin (1 mg / ml) and leupeptin (100 μM). )Resuspended. Distill the suspension at 4 0 C sonication and at 4 0 C with 12,000xg (14,600rpm, SS34 rotor) centrifugation 20min after recovering cell lysate to form the crude flake precipitate of cell debris, nucleus, peroxisome, lysosome and mitochondria; Supernatants or cell lysates were used for FAAH enzyme assays. In some cases, by subjecting cell lysates to 4 0 C was...
Embodiment A71
[0533] 2-(5-{5-[(5-Chloropyridin-2-yl)thio]-2-(tetrahydro-2 H -pyran-4-yl)-1,3-oxazol-4-yl}pyridin-2-yl)propan-2-ol
[0534]
[0535] Oxazole 5-bromide A6.1 (172 mg, 0.467 mmol), 5-chloropyridine-2-thiol (136 mg, 0.934 mmol), K 3 PO 4 (297 mmol, 1.40 mmol), N,N- A mixture of dimethylglycine (9.6 mg, 0.093 mmol) and CuI (18 mg, 0.093 mmol) in DMF (4.67 mL) was heated at 145 °C for 5 h. Using reverse phase HPLC (C-18, 35-95% MeCN in H 2 O, containing 0.05% TFA) to give the product. 1 H NMR (CDCl 3 , 400 MHz)δ9.15 (bs, 1H), 8.39 (d, J = 2.8 Hz, 1H), 8.32 (d, J = 8.4 Hz, 1H), 7.54 (dd, J = 3.0, 8.4 Hz, 1H), 7.42 (d, J = 7.6 Hz, 1H), 6.95 (d, J = 8.4 Hz, 1H), 4.84 (bs, 1H), 4.06 (td, J = 3.6, 8.4 Hz, 2H), 3.57 (dt, J = 3.2, 11.6 Hz, 2H), 3.17 (m, 1H), 2.10-1.97 (m, 4H), 1.55 (s, 6H). C 21 h 23 ClN 3 o 3 S HRMS (ES) [M+1] + Calculated: 432.1143, Found: 432.1141.
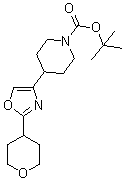
[0536] Intermediate A6.2
[0537]
[0538] tert-butyl 4-{5-bromo-4-[4-(methylsulfinyl...
Embodiment A713
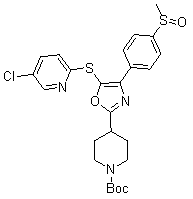
[0549] 4-{5-[(5-chloropyridin-2-yl)thio]-4-[4-(methylsulfinyl)phenyl]-1,3-oxazol-2-yl}piperidine- tert-Butyl 1-formate
[0550]
[0551] Intermediate 6.2 (685 mg, 1.46 mmol), 5-chloropyridine-2-thiol (531 mg, 3.65 mmol) and potassium carbonate (605 mg, 4.38 mmol) were dissolved in NMP (14.6 mL) and the resulting solution Heat to 85 oC for 16 hours in a closed tube. The solution was then cooled to 25 °C, diluted with ethyl acetate, washed with aqueous lithium chloride (x3), dried over sodium sulfate and concentrated in vacuo. The crude oil was purified by silica gel chromatography (100 g using a gradient of 25-100% ethyl acetate in hexanes) to afford 658 mg of the desired product as a clear oil. LCMS (M+1) = 534.5. 1 H NMR (CDCl 3 ): δ8.38 (d, J = 2.1 Hz, 1H), 8.21 (d, J = 6.8 Hz, 2H), 7.7 (d, J = 6.8 Hz, 2H), 7.54 (dd, J = 8.5 Hz, 2.1 Hz, 1H), 6.94 (d, J = 8.5 Hz, 1H), 4.15 (m, 2H), 3.1 (m, 3H), 2.73 (s, 3H), 2.15 (m, 2H), 1.95 (m, 2H), 1.51 (s, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com