Method for preparing nano ferrate in fused salt manner
A technology of ferrite and molten salt method, which is applied in the direction of nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of poor product crystallinity, increased cost and environmental pollution, and achieve rich product types and short reaction time , highly controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0035] This example is MgFe 2 o 4 Preparation method of nanospheres:
[0036] Put 1mmol magnesia, 2mmol ferric nitrate, 100mmol sodium chloride, 10mmol potassium chloride to grind, mix evenly, put the mixture in an alumina crucible, then put the alumina crucible into a tube furnace, press 5°C / min Raise the temperature to 820°C and bake for 4 hours.
[0037] Cool naturally to ambient temperature (0-40°C, the same below), immerse in distilled water to remove salt, filter with suction, wash, and dry at 70°C to obtain MgFe 2 o 4 nanospheres.
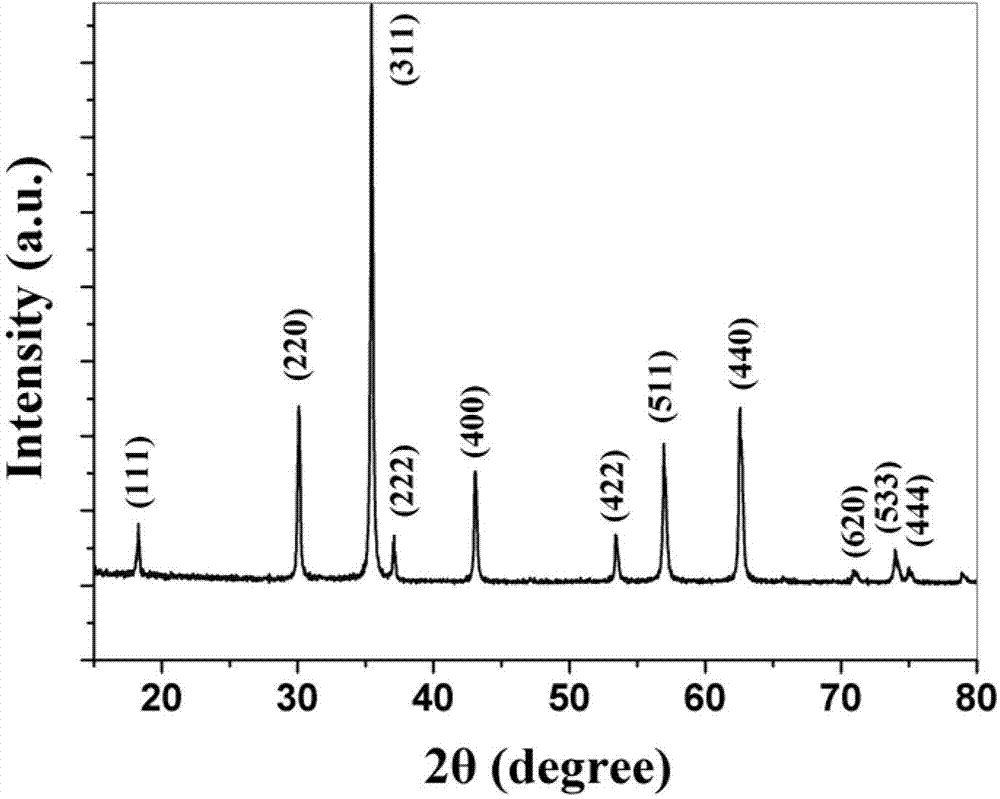
[0038] The MgFe 2 o 4 The X-ray diffraction pattern of the nanospheres is shown in figure 1 ,Depend on figure 1It can be seen that the X-ray diffraction peak position of this product is consistent with the standard X-ray diffraction card (JCPDS 36-0398), which proves that the product obtained in this example is pure phase MgFe 2 o 4 powder.
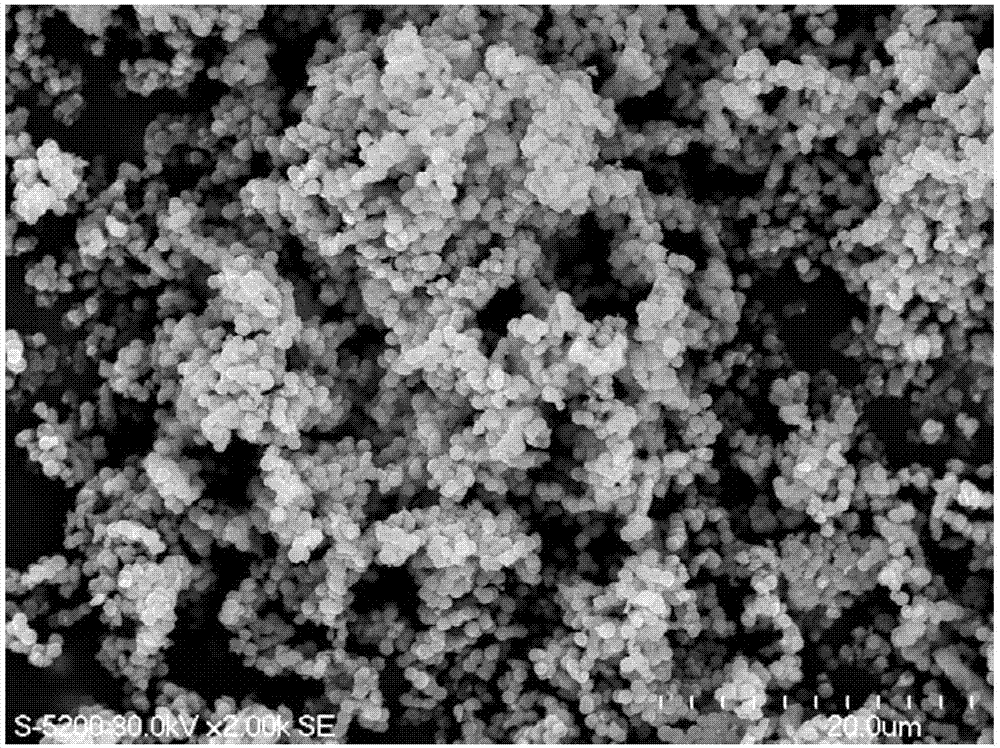
[0039] The MgFe 2 o 4 The field emission scanning electron microscope image of the nano...
Embodiment 2)
[0042] This example is PbFe 2 o 4 Preparation method of nanospheres:
[0043] Put 2mmol lead carbonate, 4mmol ferric nitrate, 100mmol sodium chloride, 10mmol potassium chloride to grind, mix evenly, place the mixture in an alumina crucible, then put the alumina crucible into a tube furnace, pass in an inert gas, and Under the protection of inert gas, the temperature was raised to 840°C at a rate of 5°C / min, and calcined for 4h.
[0044] Naturally cool to ambient temperature, impregnate with distilled water to remove salt, filter, wash, and dry at 60°C to obtain PbFe with a particle size of 800nm to 1000nm 2 o 4 nanospheres.
[0045] Control the molar ratio of divalent metal to ferric iron to be 1:2, the molar ratio of raw material to molten salt to be 3:11y (0.6≤y2 o 4 , SnFe 2 o 4 nanospheres.
Embodiment 3)
[0047] This example is Ni 0.6 Fe 2.4 o 4 Preparation method of nanospheres:
[0048] Put 0.6mmol nickel nitrate, 2.4mmol ferric nitrate, 80mmol sodium chloride, 8mmol potassium chloride to grind, mix evenly, put the mixture in an alumina crucible, then put the alumina crucible into a tube furnace, press 5°C The temperature was raised to 860°C at a rate of 1 / min, and roasted for 6h.
[0049] Naturally cooled to ambient temperature, soaked in distilled water to remove salt, filtered, washed, and dried at 60°C to obtain Ni 0.6 Fe 2.4 o 4 nanospheres.
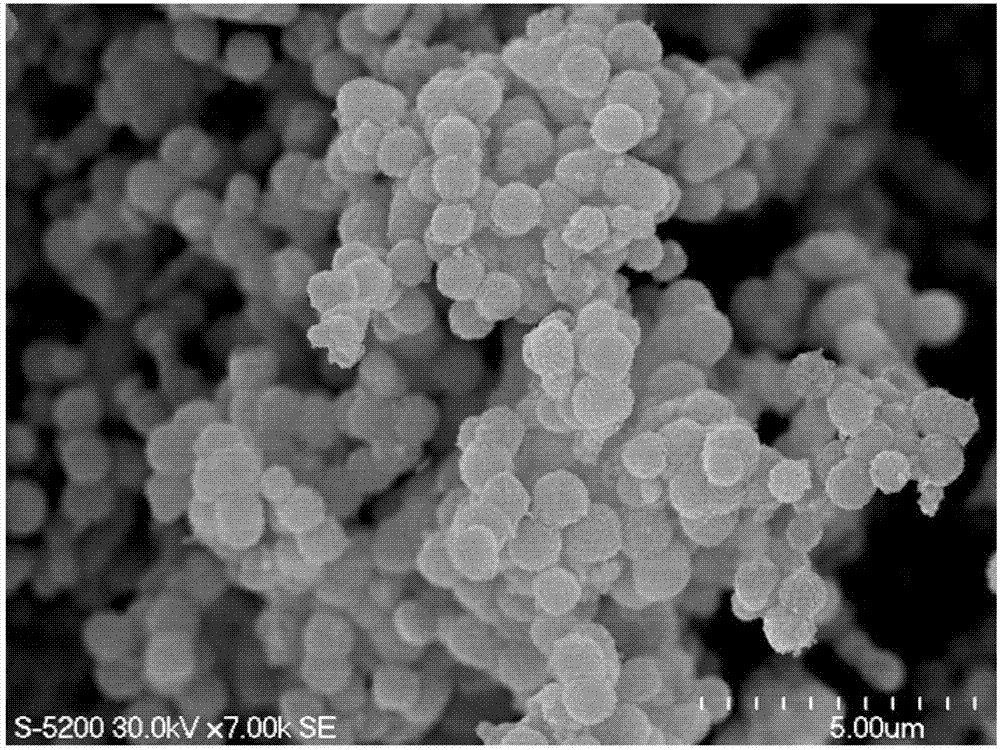
[0050] The Ni 0.6 Fe 2.4 o 4 The field emission scanning electron microscope image of the nanosphere is shown in image 3 ,Depend on image 3 It can be seen that the product has a spherical shape with a diameter of about 600nm to 1100nm, Ni 0.6 Fe 2.4 o 4 The yield of nanospheres was about 80 wt%.
[0051] Control the molar ratio of divalent metal and ferric iron to x:3-x (0.01≤xx Fe 3-x o 4 、Cu x Fe 3-x o 4 、Cd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com