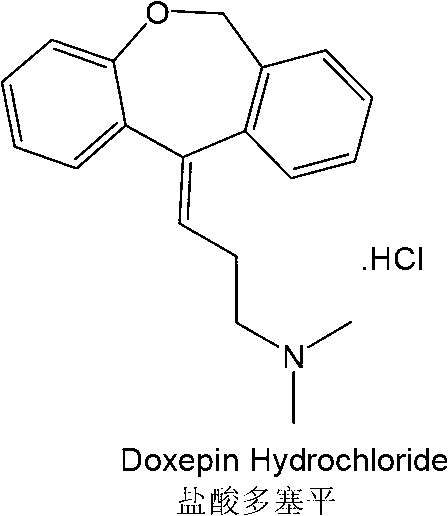
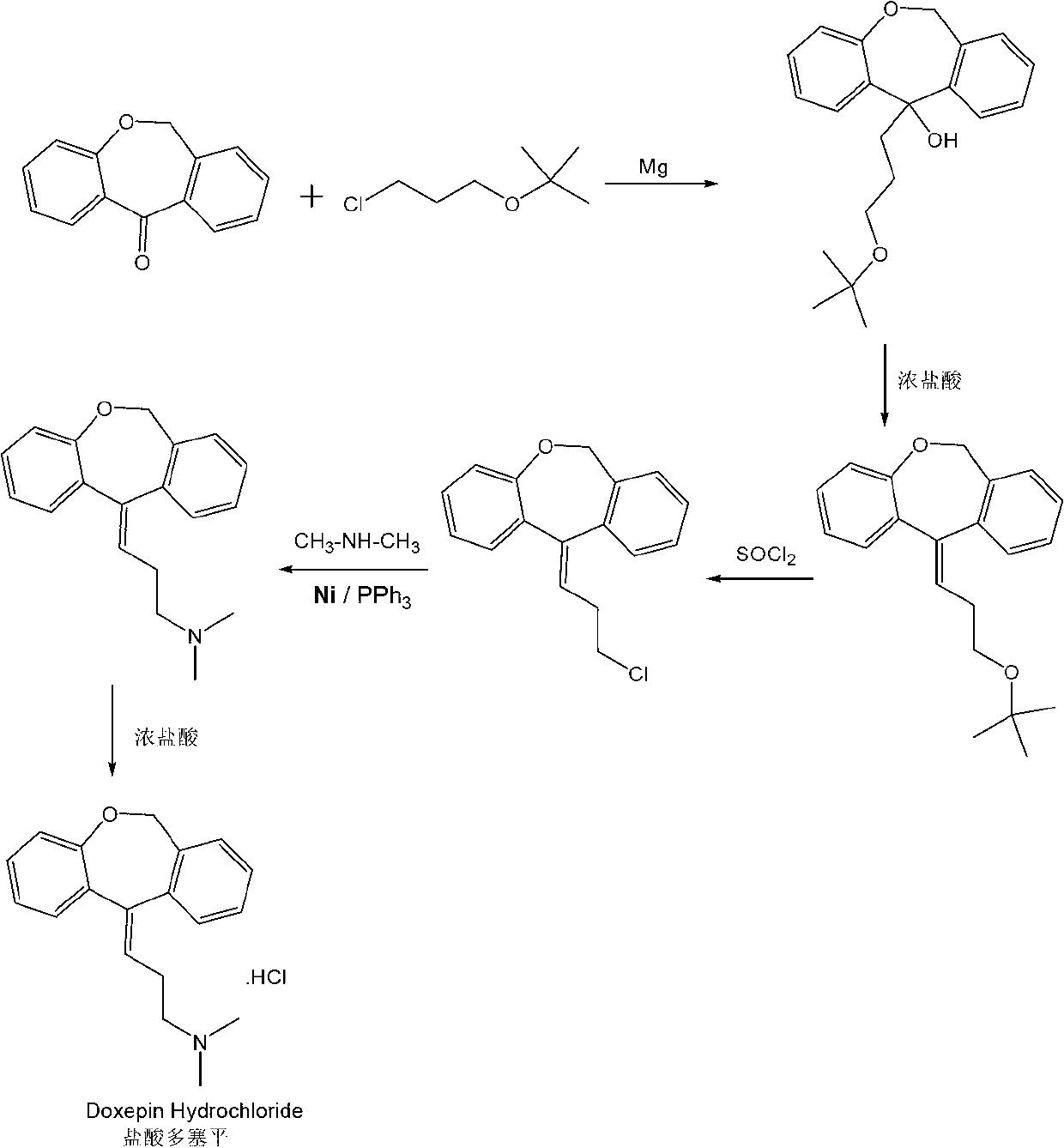
Method for synthesizing doxepin hydrochloride
A technology of doxepin hydrochloride and doxepin, applied in chemical recovery, organic chemistry, etc., can solve the problems of high catalyst cost, cumbersome operation, and environmental pollution of catalyst recycling, and achieve high reaction yield and good selectivity , The effect of the scientific and reasonable reaction path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1)
[0036]
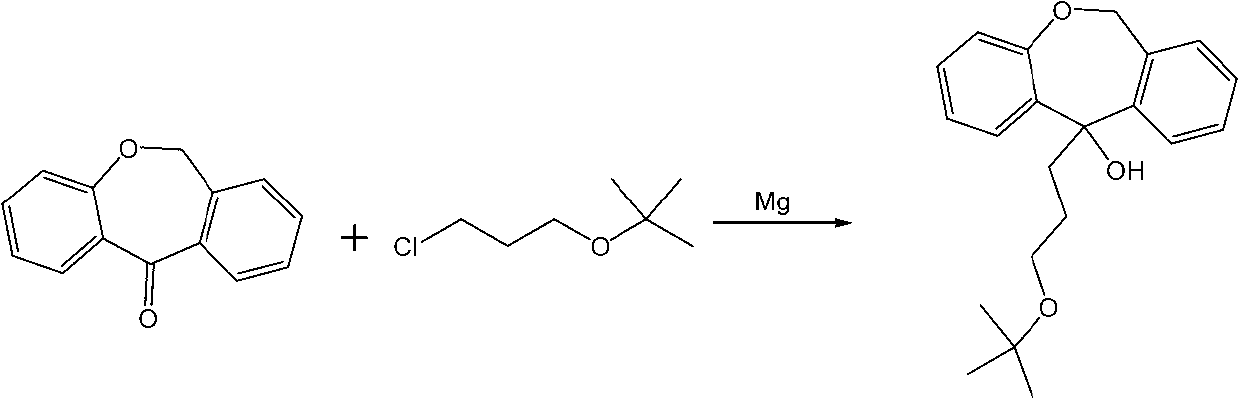
[0037] Add 0.8 liters of anhydrous tetrahydrofuran and 143 g of magnesium powder, 500 g of cyclic ketone raw materials and 895 g of 3-chloropropyl-tert-butyl ether into a 2-liter reactor, and drop 0.4 liters of anhydrous ether in half an hour Add it to the reactor and keep the system in a slightly boiling state. After the dropwise addition, argon gas is introduced into the system, and the reflux continues for 2 hours. After the reaction was completed, the system was cooled and poured into saturated ammonium chloride solution, extracted three times with ethyl acetate, dried with anhydrous sodium sulfate for 5 hours, and the obtained crude product was recrystallized with petroleum ether to obtain 698 g of the product, and the HPLC purity analysis was >99%. The yield is 90%. Product Molecular Formula: C 21 h 26 o 3 ,Molecular weight: 326.43. Melting point: 124-126°C, LC-MS: 326.46, elemental analysis: C 77.27%, H 8.03%, O 14.70%.
[0038] (2)
[...
Embodiment 2
[0051] (1)
[0052]
[0053] Add 2 liters of anhydrous tetrahydrofuran and 280g of magnesium powder, 1Kg of cyclic ketone raw material and 1.9Kg of 3-chloropropyl-tert-butyl ether into a 4-liter reactor, and divide 1 liter of anhydrous ether for half an hour Add it dropwise into the reactor, keep the system in a slightly boiling state, after the dropwise addition, feed argon into the system, and continue to reflux for 2h. After the reaction was completed, the system was cooled and poured into a saturated ammonium chloride solution, extracted three times with ethyl acetate, dried with anhydrous sodium sulfate for 5 hours, and the obtained crude product was recrystallized with petroleum ether to obtain a product of 1.42Kg, HPLC purity analysis >99% , yield 88%. Product Molecular Formula: C 21 h 26 o 3 ,Molecular weight: 326.43. Melting point: 124-126°C, LC-MS: 326.46, elemental analysis: C 77.27%, H 8.03%, O 14.70%.
[0054] (2)
[0055]
[0056] Add 1.4Kg of the ...
Embodiment 3
[0067] Compared with embodiment 1, the difference of this embodiment only lies in:
[0068] Step 1 is: 6,11-dihydrodibenzo[b,e]oxepin-11-one and 3-chloropropyl-tert-butyl ether with a molar ratio of 1:1.2 in the absence of catalyst Addition reaction was carried out in water and ether, and the reaction was refluxed to obtain alcohol compounds. Wherein, anhydrous diethyl ether is added dropwise as a solvent, and its volumetric dosage is 5.8 times the weight of 6,11-dihydrodibenzo[b,e]oxepin-11-one, and its dropping speed is 5% of its dosage per minutes, the catalyst is magnesium powder, the dosage of magnesium powder is twice that of 6,11-dihydrodibenzo[b,e]oxepin-11-one, the reaction time is 3h, and the temperature is 38°C .
[0069] In step 2, the alcohol compound is kept boiling under the condition of concentrated hydrochloric acid, and the amount of concentrated hydrochloric acid is 6 times that of the reaction substrate; the reaction time is 6 hours.
[0070] In step 3, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com