Application of ferric iron ferriporphyrin compound in preparation of anti-type-2 diabetes drug
A technology of type 2 diabetes and ferric iron, applied in the direction of drug combination, dipeptide component, tetrapeptide component, etc., to achieve the effect of lowering blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Effect of D1 to D10 on promoting sugar consumption in C2C12 cells
[0032] After administration, the glucose content in the culture supernatant of differentiated C2C12 cells was determined by the reductase method, and the effect of the drug on promoting the glucose consumption of C2C12 cells was observed.
[0033] experimental method:
[0034] 1. Cell line: C2C12, a mouse skeletal muscle cell line;
[0035] 2. The final concentration of the drug acting on C2C12 cells is 16 μM;
[0036] 3. When the cells proliferate to 80%~90% in the culture flask, digest with 0.25% trypsin, centrifuge, add medium and blow gently to form a cell suspension, count, 10 4 Place each well in a 96-well plate, change the medium every 2 days, add inducing factors to induce C2C12 cells to differentiate into myotube cells when the cell proliferation reaches 70%, change the low-glucose medium overnight, and change the low-glucose medium again the next morning Different drugs were admi...
Embodiment 2
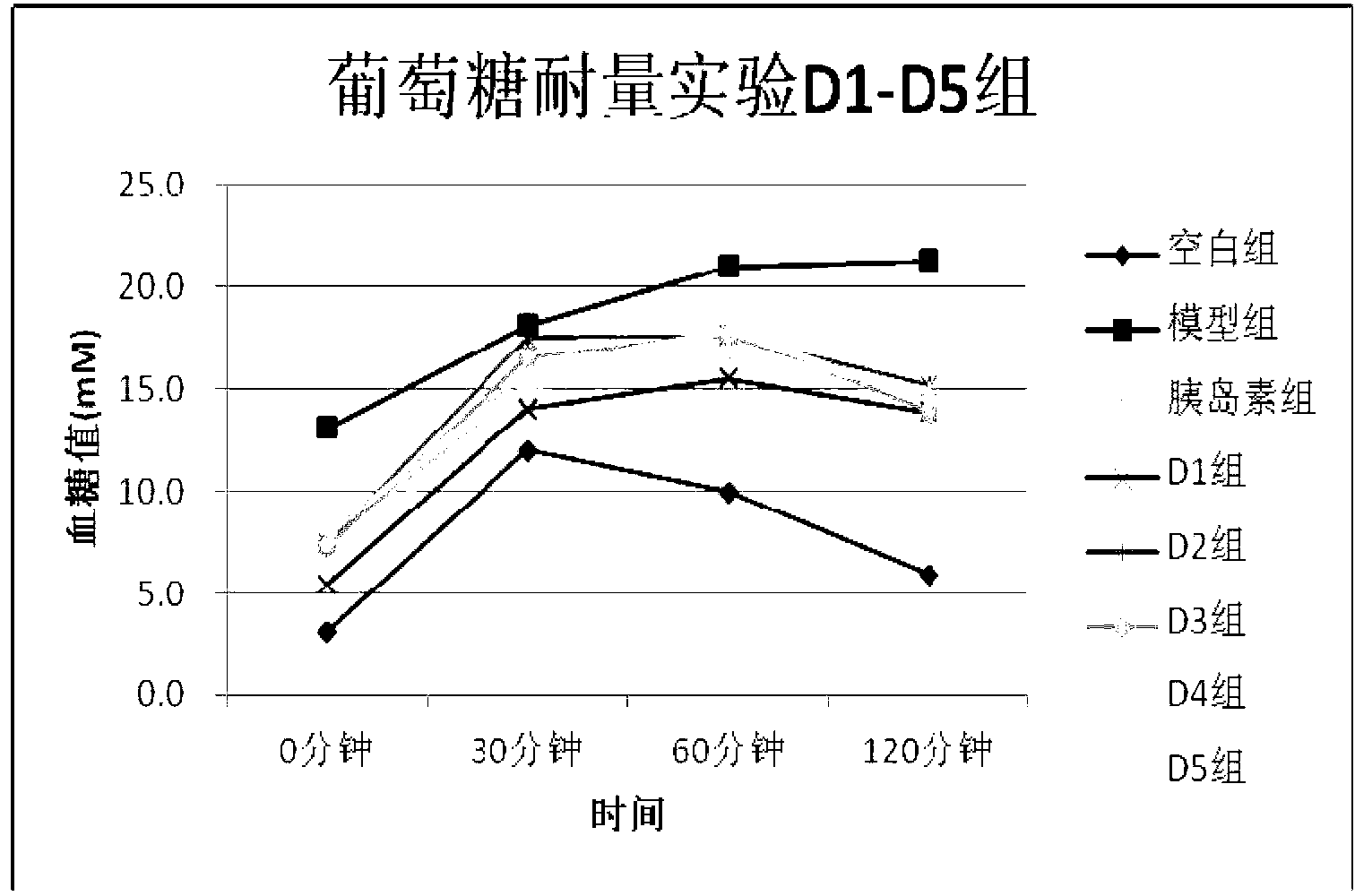
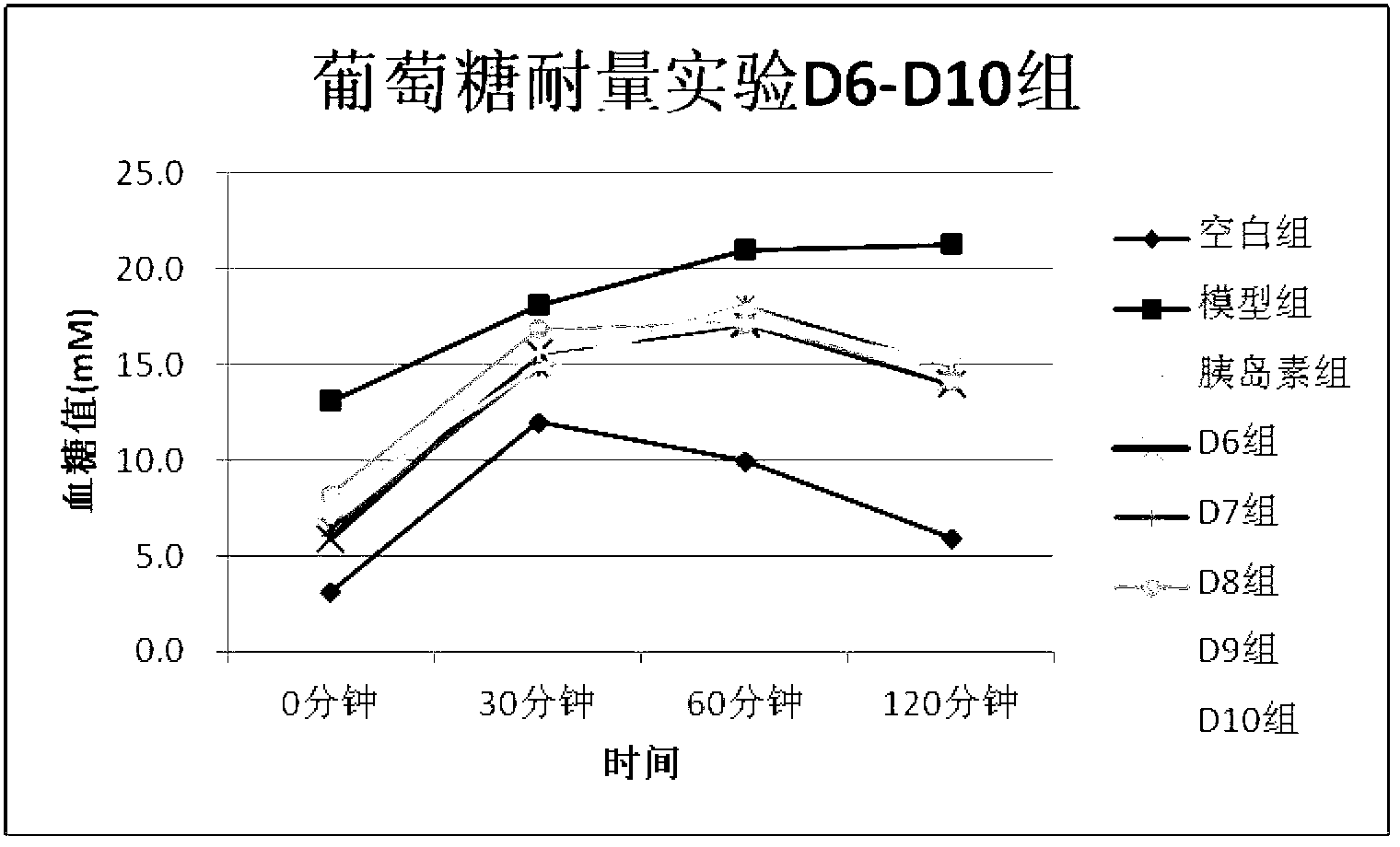
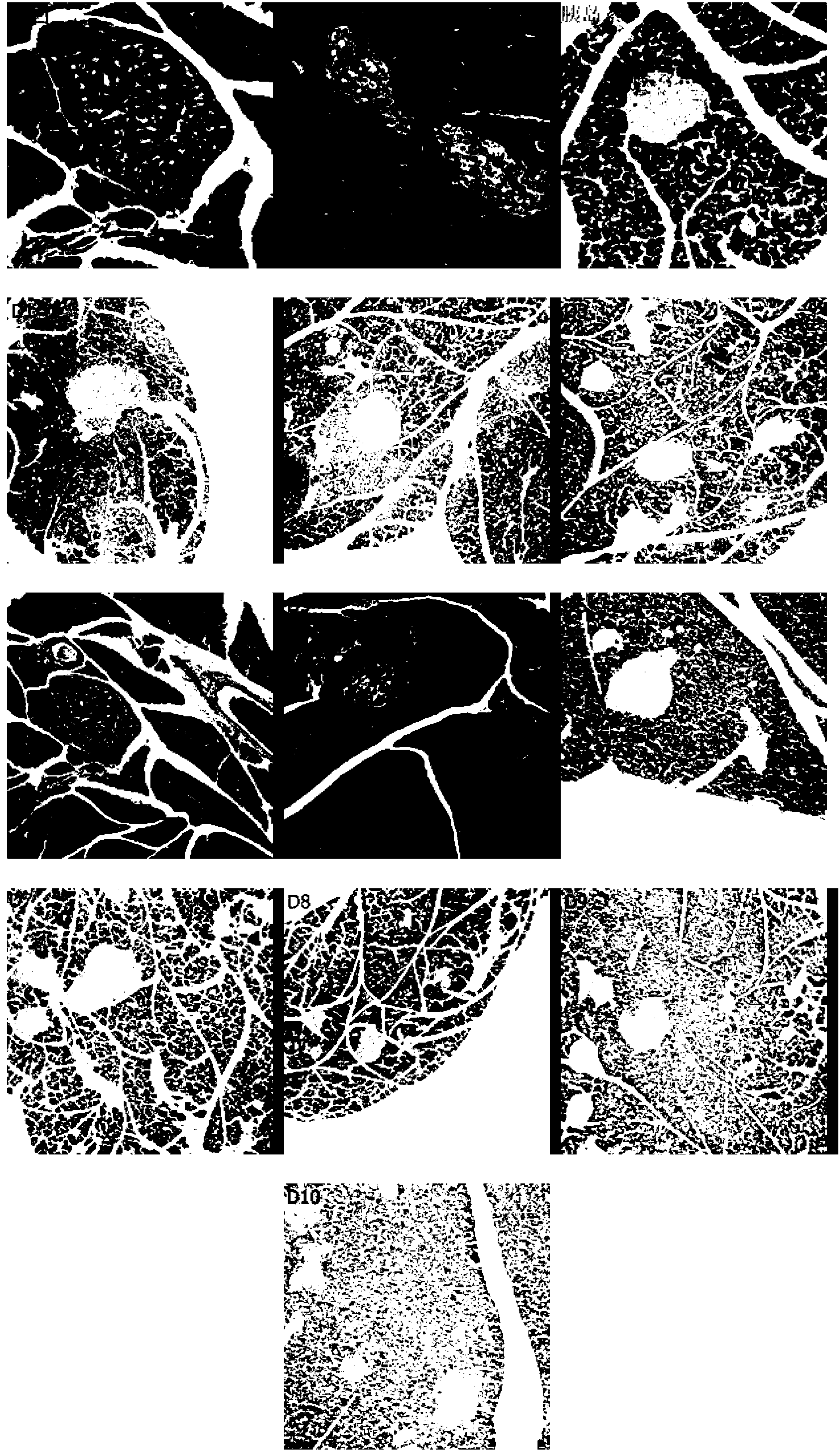
[0043] Example 2 Observation of the effects of D1~D10 drugs on blood sugar in type 2 diabetic rats induced by high fat and STZ
[0044] experimental method:
[0045] After feeding rats with high-fat diet for 4 weeks, 30 mg / kg STZ was injected intraperitoneally twice to prepare diabetes models, and the model rats with fasting blood glucose in the range of 7.8-15.0 were selected into groups, normal control group, model group, insulin treatment group (subcutaneous injection ,3.2U / kg), the drug treatment (subcutaneous injection 0.3mg / kg) group. After subcutaneous injection for 3 weeks, fasting blood glucose, glucose tolerance test, fasting insulin test, fasting cholesterol test, and fasting triglyceride test were performed.
[0046] Experimental results:
[0047] After 3 weeks of subcutaneous injection in each drug group from D1 to D10, the fasting blood glucose in the drug group and insulin group was significantly lower than that in the model group (see Table 2). significantly...
Embodiment 3
[0054] Embodiment 3 toxicity test
[0055] 1. Acute toxicity test
[0056] A single intravenous injection of D4 was given to ICR mice, and the symptoms of poisoning, degree of poisoning, nature, recovery and death were observed to clarify the acute toxicity of the drug and to understand the target organs of acute toxicity. Adverse reaction monitoring provides reference materials.
[0057] experiment method:
[0058] In this experiment, the median lethal dose method was used. There are 7 dosage groups for males and females, and the dosages for each group are 49, 70, 100, 143, 204, 292, 417 mg·kg-1 respectively; 10 mice in each group are administered intravenously, and the administration volume is 0.25ml / 10g, observed for 14 days after administration. The poisoning symptoms, appearance and duration of the animals were observed, the development of the poisoning symptoms, the time of death, and the number of deaths were observed, and the dead animals were autopsyed.
[0059]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com