Antineoplastic prodrug with P-glycoprotein inhibition function
An anti-tumor drug, glycoprotein technology, applied in the direction of anti-tumor drugs, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of slow drug release, enhanced therapeutic effect, and no special effects. and other problems, to achieve the effects of small steric hindrance, long cycle, improved solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
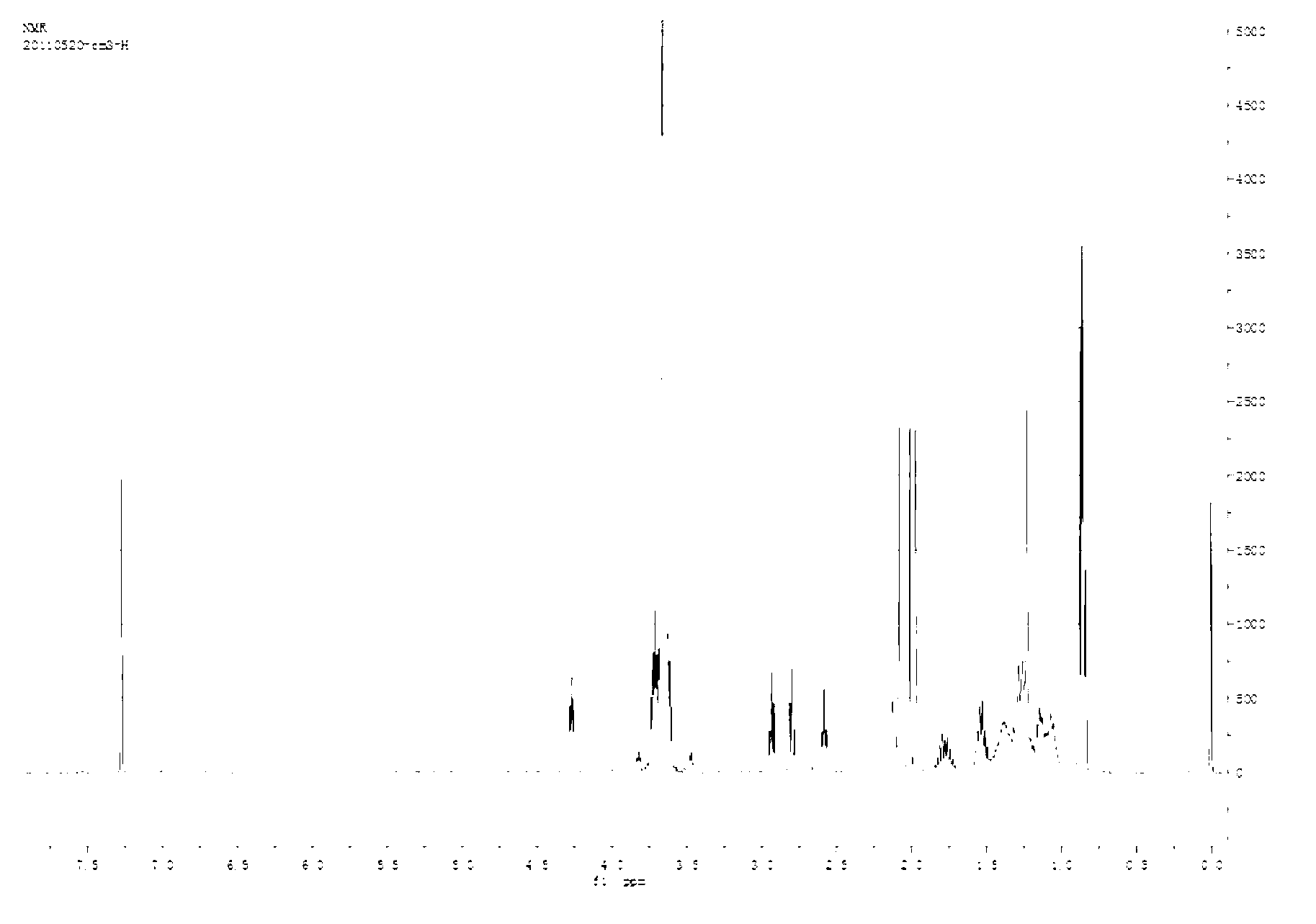
Embodiment 1
[0045] This embodiment provides a paclitaxel prodrug with P-glycoprotein inhibitory function, which is synthesized through the following steps:
[0046] (1) Synthesis of 3,3'-dithiodipropionic anhydride: Weigh 2g of 3,3'-dithiodipropionic acid into a 50ml round bottom flask, add 20ml of acetyl chloride, and reflux at 65°C for 3 hours, distilled under reduced pressure to remove impurities such as acetic acid and acetyl chloride; washed three times in diethyl ether, and evaporated diethyl ether under reduced pressure to obtain 3,3'-dithiodipropionic anhydride.
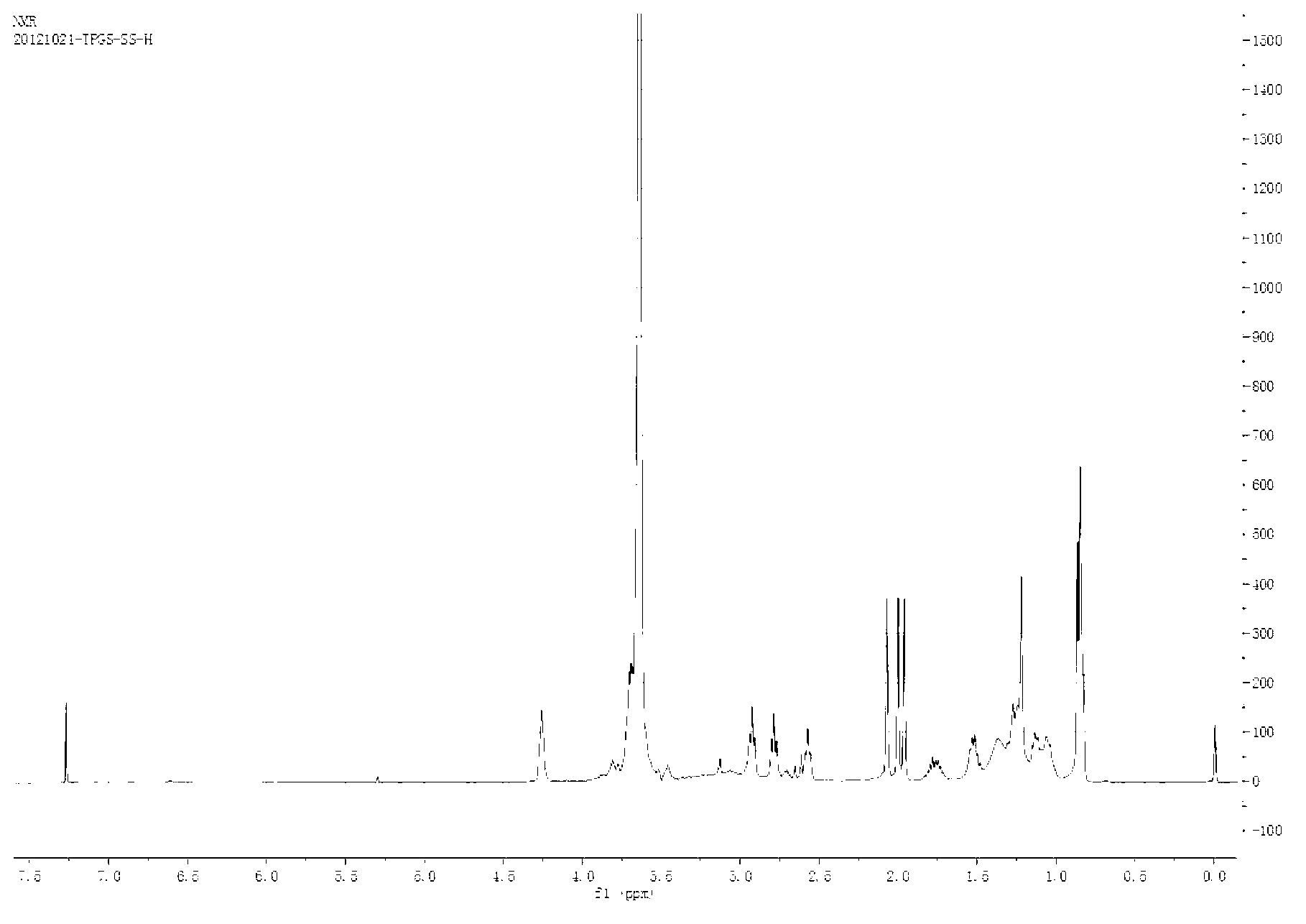
[0047] (2) Synthesis of TPGS-S-S-COOH: Weigh 1.5g TPGS into a 100mL round-bottomed flask, dry in a vacuum oven at 60°C for 3-5 hours, and then add 0.276g 3,3'-COOH Thiodipropionic anhydride, 0.183g 4-dimethylaminopyridine, 0.15mL triethylamine, dissolved in 5-10mL dimethyl sulfoxide; in an anhydrous environment, stirred at room temperature for 24 hours.
[0048] (3) Activation of TPGS-S-S-COOH: Dialyze the above product i...
Embodiment 2
[0053] This embodiment provides a doxorubicin prodrug with P-glycoprotein inhibitory function, which is synthesized through the following steps:
[0054] (1) Obtain activated TPGS-S-S-COOH in the same manner as in the first three steps of Example 1.
[0055] (2), connect doxorubicin: add 0.6g doxorubicin in a 100mL round bottom flask, add 5-10mL DMSO and 0.12ml triethylamine to dissolve, and do light-proof treatment; the product of step (1) Filtrate, add the filtrate to the doxorubicin solution; stir at room temperature for 48 hours in an anhydrous environment; use a dialysis bag with a molecular weight of 2000 to dialyze in a mixed solvent of absolute ethanol and DMSO for 24-48 hours, and evaporate the ethanol to dryness after the dialysis That is doxorubicin prodrug (TPGS-S-S-DOX), its structure is as follows:
[0056]
Embodiment 3
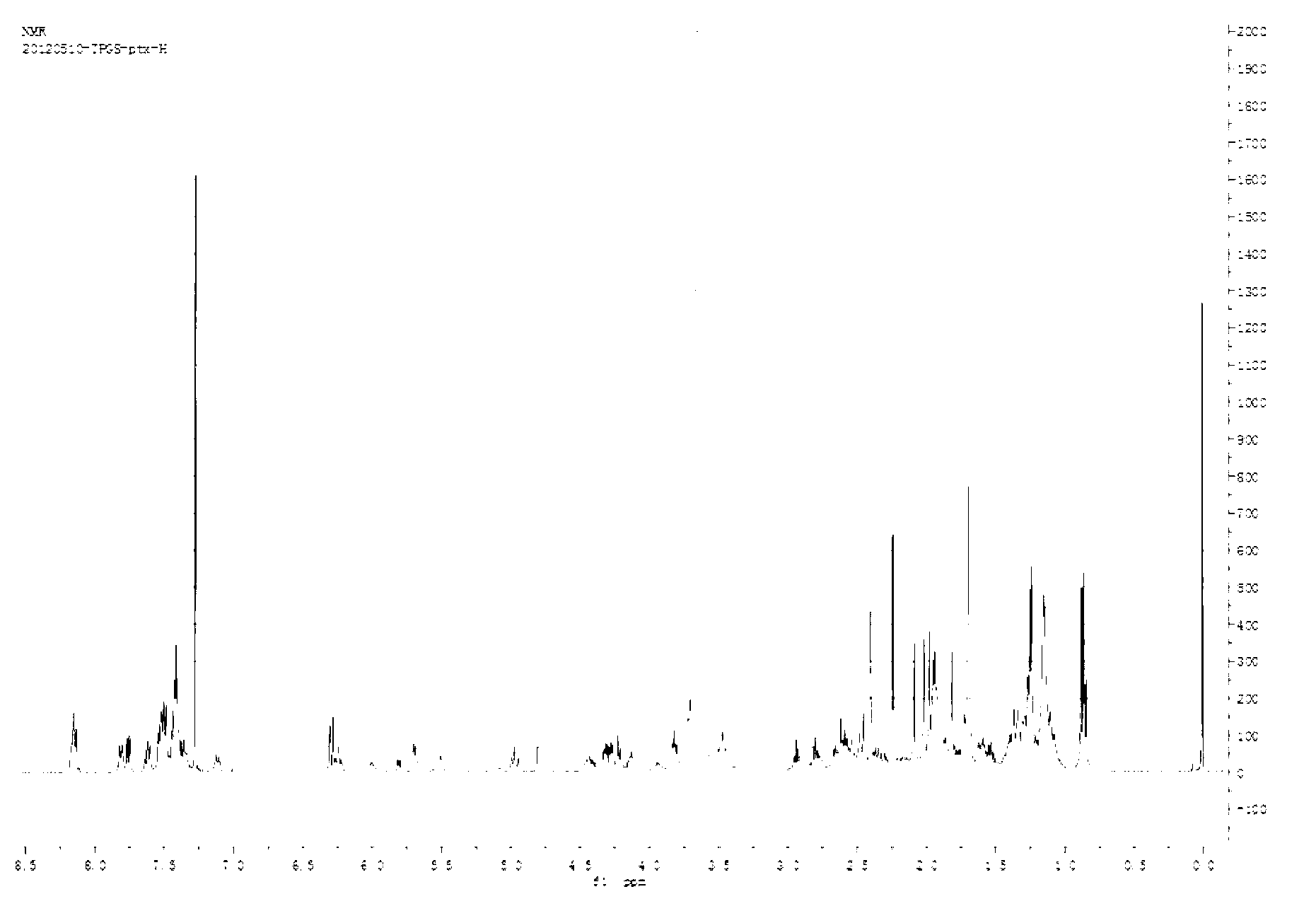
[0058] This embodiment provides a paclitaxel prodrug with P-glycoprotein inhibitory function, which is synthesized by the following steps:
[0059] (1) Synthesis of diselenomalonic acid: Weigh 1.5g of sodium borohydride in a 100mL round bottom flask, add 20ml of deionized water, and then add 3.2g of selenium powder; after stirring for 10 minutes, heat the solution to 70°C React until the selenium powder disappears completely; add 3-chloropropionic acid tetrahydrofuran solution (4.36g / 50ml) under the protection of nitrogen, react at 50°C for 12h, remove tetrahydrofuran by rotary evaporation, freeze-dry and wash with ether to obtain diselenide On behalf of malonic acid.
[0060] (2) Synthesis of TPGS-Se-Se-COOH: Weigh 1.5g of TPGS into a 100mL round bottom flask, dry in a vacuum oven at 60°C for 3 to 5 hours, and then add 0.306g of diselenide Acetic acid, 0.183g 4-dimethylaminopyridine, 0.15mL triethylamine, dissolved in 5-10mL DMSO; in anhydrous environment, stirred at room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com