Preparation method of 1-oxacephalosporin-3-epoxymethylene derivatives and use of the 1-oxacephalosporin-3-epoxymethylene derivatives in preparation of 1-oxacephalosporin
A technology of epoxymethylene and oxacephalosporins, applied in the production of bulk chemicals, organic chemistry, etc., to achieve the effects of reducing investment, avoiding equipment corrosion, and reducing the probability of danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
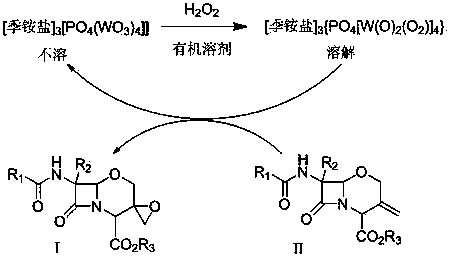
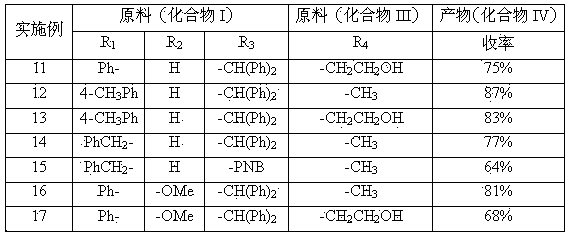
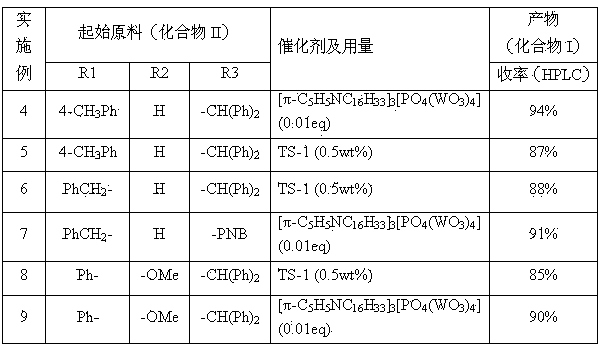
[0034] Compound II (R1 is phenyl, R2 is hydrogen atom, R3 is benzhydryl) was stirred and dissolved in 10 times the amount of methanol, and compound II weight 0.8% TS-1 molecular sieve was added at room temperature (25°C), and the control was the same 1.8 times the molar amount of compound II in 30% aqueous hydrogen peroxide solution was added dropwise at high temperature. TLC detected that the reaction was complete. The TS-1 molecular sieve catalyst was removed by filtration, and the reaction solution was concentrated to dryness. The yield was about 90% (HPLC external standard method). The residual solid was dissolved in dichloromethane, washed with a small amount of sodium thiosulfate solution, the organic layer was evaporated to dryness, and the residual solid was recrystallized with ethyl acetate:n-heptane (4:1) to obtain off-white compound I (R1 is phenyl, R2 is a hydrogen atom, R3 is a benzhydryl group). Yield 61%. 1 H-NMR (300MHz, CDCl 3 ): δ 8.45 (brs, 1H), 7.72~7.94 ...
Embodiment 2
[0036] Compound II (R1 is a phenyl group, R2 is a hydrogen atom, R3 is a benzhydryl group) was stirred and dissolved in 10 times the amount of methanol, and 0.01 times the molar amount of the compound II was added at room temperature (25°C) [π-C 5 h 5 NC 16 h 33 ] 3 [PO 4 (WO 3 ) 4 ] catalyst, under the control of the temperature, dropwise add 1.2 times the molar amount of compound II in 30% aqueous hydrogen peroxide solution, TLC detects that the reaction is complete, filter and remove the precipitated heteropolytungstate quaternary ammonium salt catalyst, and concentrate the reaction solution to dryness to obtain compound I (R1 is phenyl, R2 is a hydrogen atom, R3 is a benzhydryl group), and the yield is about 95% (HPLC). The reaction product can be directly used in the next reaction without further purification.
Embodiment 3
[0038] Compound II (R1 is a phenyl group, R2 is a hydrogen atom, R3 is a benzhydryl group) was stirred and dissolved in 10 times the amount of dichloromethane, and 0.05 times the molar amount of the compound II was added at room temperature (25°C) [C 18 h 37 N(CH 2 Ph)(CH 3 ) 2 ] 3 [PO 4 (WO 3 ) 4 ] catalyst, under the control of the temperature, dropwise add 1.2 times the molar amount of compound II in 30% aqueous hydrogen peroxide solution, TLC detects that the reaction is complete, filter and remove the precipitated heteropolytungstate quaternary ammonium salt catalyst, and concentrate the reaction solution to dryness to obtain compound I (R1 is phenyl, R2 is a hydrogen atom, R3 is a benzhydryl group), and the yield is about 91% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com