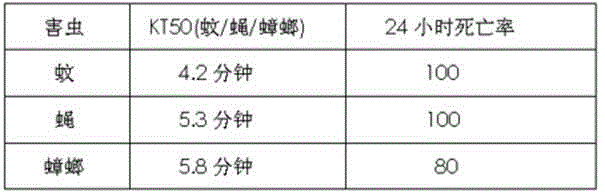
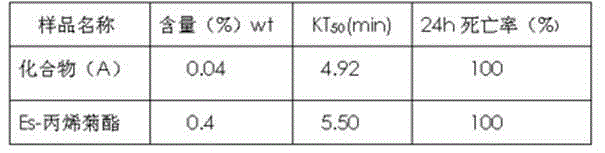
Pyrethroid compound as well as preparation method and application thereof
A technology for pyrethroids and compounds is applied in the field of pyrethroid compounds, which can solve the problems of obvious irritation to humans and animals and high toxicity, and achieve the effects of easy industrialization, improved quality and simple technology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
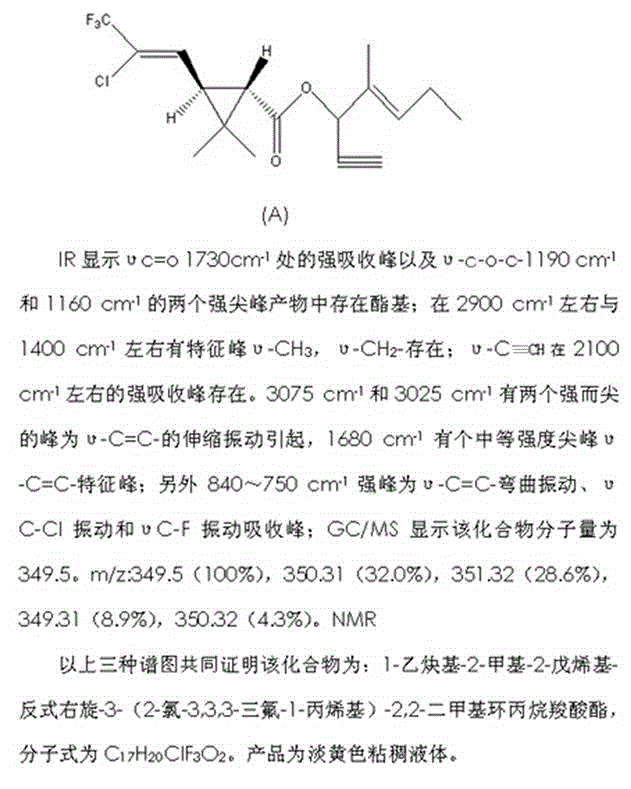
[0044] Preparation Example 1: Resolution of trans-3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid
[0045] In a 500ml four-necked bottle, put 200ml of water, 24.0g of sodium carbonate, racemic trans-3-(2-chloro-3,3,3-trifluoroallyl)-2,2-dimethyl 50.0g of cyclopropane carboxylic acid, stir to dissolve, control the temperature at 20-25°C, add dropwise a solution of 21.0g of D-phenylglycine ethyl ester hydrochloride dissolved in 100ml of water, add dropwise for 1 hour, keep stirring for 1 hour, at this time A white solid was precipitated, filtered by suction, washed with 100 ml of water, and dried by suction. The obtained solid was added to 100ml of 5% hydrochloric acid and stirred to precipitate crystals, filtered by suction, washed with 100ml of water, and dried to obtain trans-dex-3-(2-chloro-3,3,3-trifluoro-1-propenyl )-2,2-dimethylcyclopropanecarboxylic acid 20.0g, dextrorotatory effective body ee value 96%.
preparation Embodiment 2
[0046] Preparation Example 2: Chloroacylation of trans-dex-3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid
[0047] In a 500ml four-necked bottle, put the trans-dex-3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethyl obtained in Preparation Example 1 Cyclopropanecarboxylic acid 121.2g, (0.5mol, ee value 96%), dissolved in 200ml toluene, stirred evenly, heated to 40-60°C, added SOCl dropwise 2 72g, (0.6mol), dripped within 2 hours, then raised the temperature to 65°C, and kept the temperature for 1 hour. Heating to 80°C under 0.08MPa negative pressure to remove solvent toluene, then rectifying under 0.09MPa negative pressure, collecting fractions at 70-75°C to obtain trans-dex-3-(2-chloro-3,3,3 -Trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid chloride 123.6g, content 95.2%, yield 90.5%, dextro-trans effective body ee value 96%.
preparation Embodiment 3
[0048] Preparation Example 3: 1-ethynyl-2-methyl-2-pentenyl-(trans-dextrorotatory)-3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-di Synthesis of Methylcyclopropane Carboxylate.
[0049] In a 1000ml four-neck flask, put 500g of toluene, 56g of 1-ethynyl-2-methylpenten-2-ol (0.45mol), and 50.0 of pyridine, stir evenly, cool down to 5°C, and prepare dropwise Trans-dex-3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid chloride 123.6g (0.45mol) obtained in Example 2 , added dropwise for 2 hours, the dropwise addition was completed, and the reaction was completed at 20° C. for 6 hours. Add 300ml of 5% hydrochloric acid to wash once, add 300ml of water to wash twice, and remove the solvent from the reaction solution under a negative pressure of 0.09MPa. 1-Ethynyl-2-methyl-2-pentenyl-(trans-dextro)-3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2- Dimethylcyclopropane carboxylate, compound (A), has a weight of 153.0 g, a content of 97.0%, and a yield of 95...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com