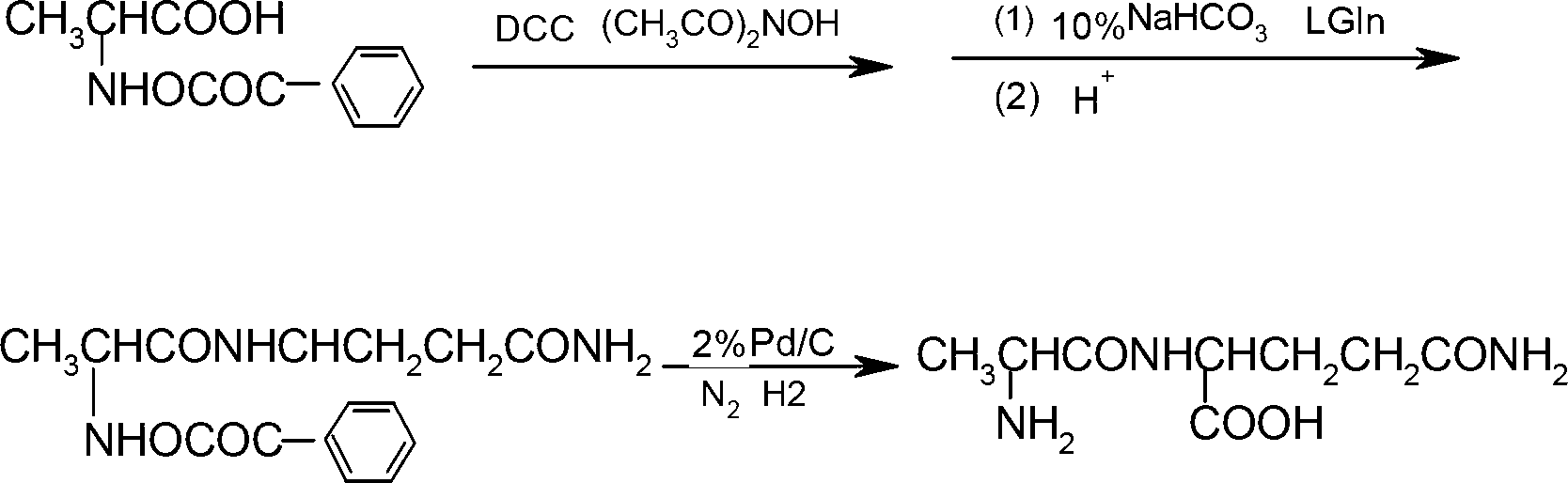
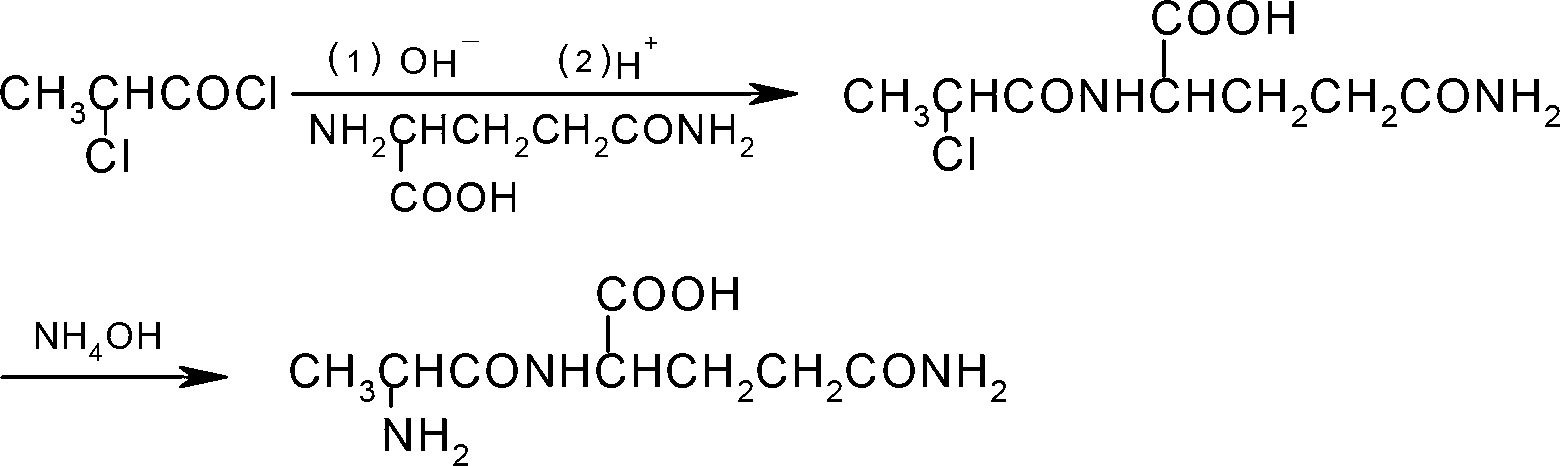
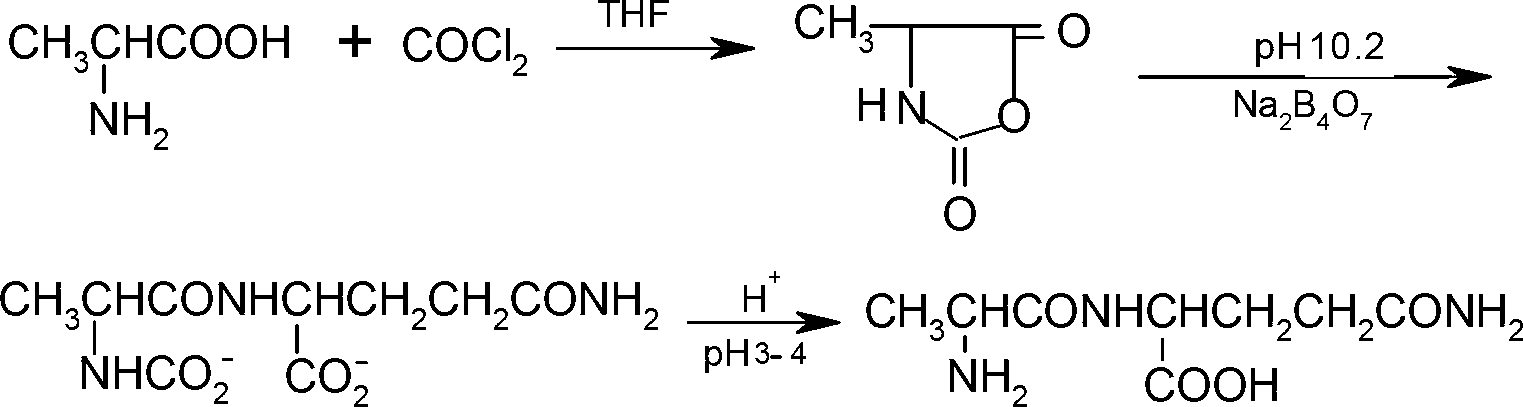
Preparation method of N(2)-L-alanyl-L-glutamine
A technology of glutamine and alanyl, which is applied in the direction of peptides, can solve the problems of difficult drying, high price, and high risk, and achieve the effects of reducing raw material costs, high production efficiency, and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A preparation method of N(2)-L-alanyl-L-glutamine, the steps are as follows:
[0047] 1) Take 87L of α-D-chloropropionic acid and 73L of thionyl chloride for heating reaction under reflux, control the temperature at 100°C-110°C, react for 5h, distill under reduced pressure, and collect the intermediate component α-D- Chloropropionyl chloride;
[0048] 2) Dissolve 127kg of L-glutamine with 5N NaOH solution, add the alkali solution of L-glutamine dropwise to the intermediate component obtained in step 1, keep the temperature at 0-5°C, and react for 3h, N (2) - α-D-chloropropionyl-L-glutamine is precipitated, and the remaining alkali is treated with hydrochloric acid;
[0049] 3) Amination reaction: Add 237kg of N(2)-α-D-chloropropionyl-L-glutamine obtained in step 2 into the ammoniation reaction tank, add 350L of ammonia water, react at 60°C for 5h, and depressurize Part of the water was removed by evaporation, and excess methanol was added to precipitate N(2)-L-alanyl-L-...
Embodiment 2
[0052] 1) Take 80L of α-D-chloropropionic acid and 50L of thionyl chloride for heating reaction under reflux, control the temperature at 105°C-110°C, react for 5h, distill under reduced pressure, and collect the intermediate component α-D- Chloropropionyl chloride;
[0053] 2) Dissolve 100kg of L-glutamine in 5N KOH solution, add the alkali solution of L-glutamine dropwise to the intermediate component obtained in step 1, keep the temperature at 0-5°C, and react for 2h, N (2) - α-D-chloropropionyl-L-glutamine is precipitated, and the remaining alkali is treated with hydrochloric acid;
[0054] 3) Amination reaction: Add 200kg of N(2)-α-D-chloropropionyl-L-glutamine obtained in step 2 into the ammoniation reaction tank, add 300L of ammonia water, react at 60°C for 5h, and evaporate under reduced pressure to remove Part of the water, add excess methanol to precipitate N(2)-L-alanyl-L-glutamine; the concentration of ammonia water used is 20%.
[0055] 4) Spray drying: use water...
Embodiment 3
[0057] 1) Take 100L of α-D-chloropropionic acid and 100L of thionyl chloride for heating reaction under reflux, control the temperature at 105°C-110°C, react for 5h, distill under reduced pressure, and collect the intermediate component α-D- Chloropropionyl chloride;
[0058] 2) Dissolve 150kg of L-glutamine in 5N NaOH solution, add the alkaline solution of L-glutamine dropwise to the intermediate component obtained in step 1, keep the temperature at 0-5°C, and react for 6h, N (2) - α-D-chloropropionyl-L-glutamine is precipitated, and the remaining alkali is treated with hydrochloric acid;
[0059] 3) Amination reaction: Add 250kg of N(2)-α-D-chloropropionyl-L-glutamine obtained in step 2 into the ammoniation reaction tank, add 500L of ammonia water, react at 80°C for 10h, and evaporate under reduced pressure to remove Part of the water, add excess methanol to precipitate N(2)-L-alanyl-L-glutamine; the concentration of ammonia water used is 10%.
[0060] 4) Spray drying: use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com