Polymersome with dual response to ultrasound and pH and method for preparing same
A technology of polymers and vesicles, applied in the fields of polymer materials and medical engineering, to achieve the effect of simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
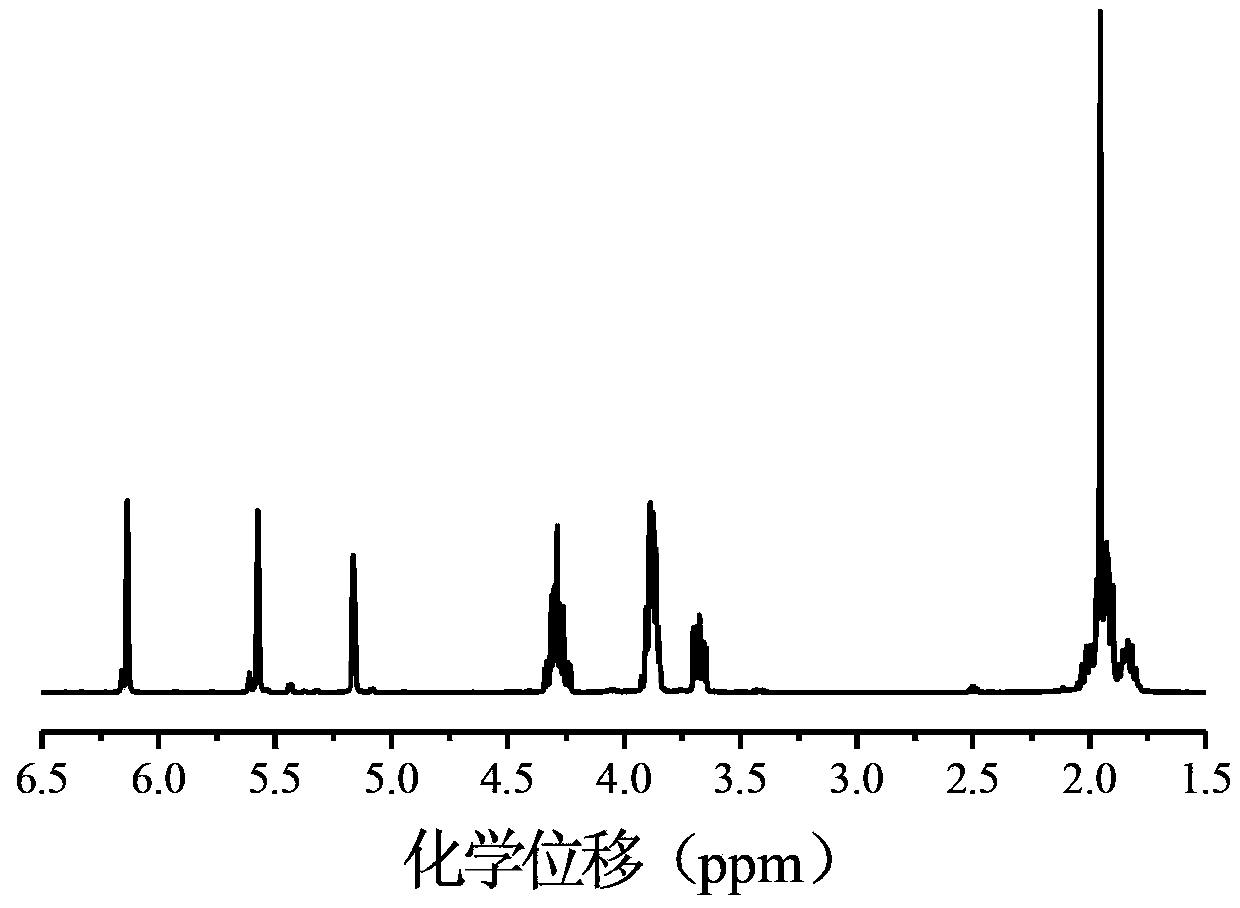
[0047] (1) Macromolecular initiator PEO 43 -Synthesis of Br
[0048] Add 10g of PEO (molecular weight: 1900) and 250mL of toluene into a 500mL round bottom flask, and remove water azeotropically at a temperature of 140°C;
[0049] After cooling to room temperature, place the flask in an ice-salt bath, add 2 mL of triethylamine, and add 20 mL of toluene solution dissolved in 2 mL of 2-bromoisobutyryl bromide dropwise at a rate of 3 seconds per drop using a constant pressure dropping funnel. After the dropwise addition, the reaction was carried out under sealed stirring for 30 hours (wherein, the molar ratio of 2-bromoisobutyryl bromide to polyethylene oxide was 2:1, and the molar ratio of triethylamine to 2-bromoisobutyryl bromide than 1:1). Filtration, extraction, washing, drying, dissolution, precipitation, and finally vacuum drying to obtain the white powdery macromolecular initiator PEO 43 -Br (molecular weight: 2025);
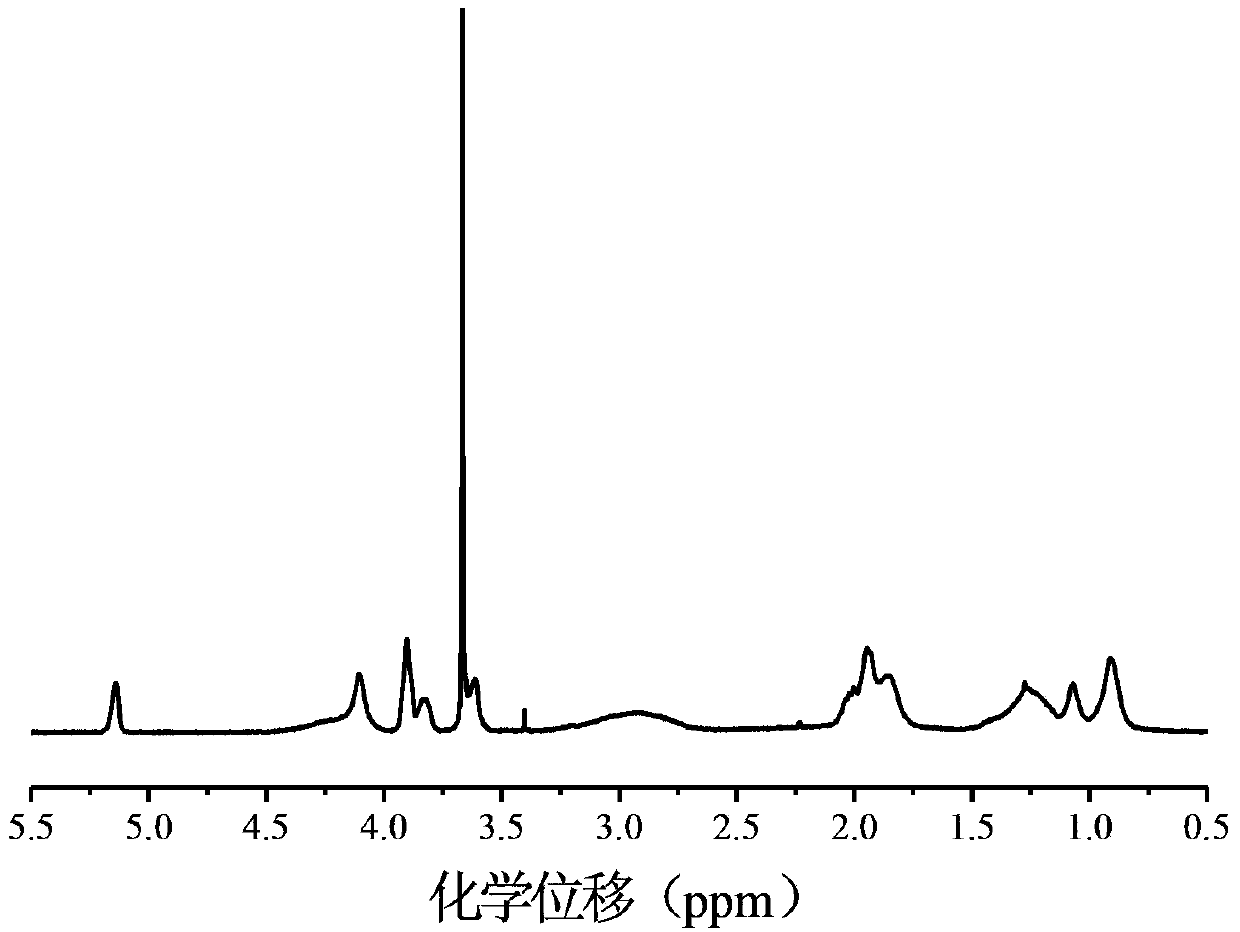
[0050] (2) Synthesis of Tetrahydrofuran Hydroxyet...
Embodiment 2
[0060] (1) Macromolecular initiator PEO 43 -Synthesis of Br
[0061] Add 10g of PEO (molecular weight: 1900) and 250mL of toluene into a 500mL round bottom flask, and remove water azeotropically at a temperature of 140°C. After cooling to room temperature, place the flask in an ice-salt bath, add 2 mL of triethylamine, and add 20 mL of toluene solution dissolved in 2 mL of 2-bromoisobutyryl bromide dropwise at a rate of 3 seconds per drop using a constant pressure dropping funnel. After the dropwise addition, the reaction was carried out under sealed stirring for 30 hours (wherein, the molar ratio of 2-bromoisobutyryl bromide to polyethylene oxide was 2:1, and the molar ratio of triethylamine to 2-bromoisobutyryl bromide than 1:1). Filtration, extraction, washing, drying, dissolution, precipitation, and finally vacuum drying to obtain the white powdery macromolecular initiator PEO 43 -Br (molecular weight: 2025).
[0062] (2) Synthesis of Tetrahydrofuran Hydroxyethyl Metha...
Embodiment 3
[0072] (1) Macromolecular initiator PEO 43 -Synthesis of Br
[0073] Add 10g of PEO (molecular weight: 1900) and 250mL of toluene into a 500mL round bottom flask, and remove water azeotropically at a temperature of 140°C. After cooling to room temperature, place the flask in an ice-salt bath, add 2 mL of triethylamine, and add 20 mL of toluene solution dissolved in 2 mL of 2-bromoisobutyryl bromide dropwise at a rate of 3 seconds per drop using a constant pressure dropping funnel. After the dropwise addition, the reaction was carried out under sealed stirring for 30 hours (wherein, the molar ratio of 2-bromoisobutyryl bromide to polyethylene oxide was 2:1, and the molar ratio of triethylamine to 2-bromoisobutyryl bromide than 1:1). Filtration, extraction, washing, drying, dissolution, precipitation, and finally vacuum drying to obtain the white powdery macromolecular initiator PEO 43 -Br (molecular weight: 2025).
[0074] (2) Synthesis of Tetrahydrofuran Hydroxyethyl Metha...
PUM
| Property | Measurement | Unit |
|---|---|---|
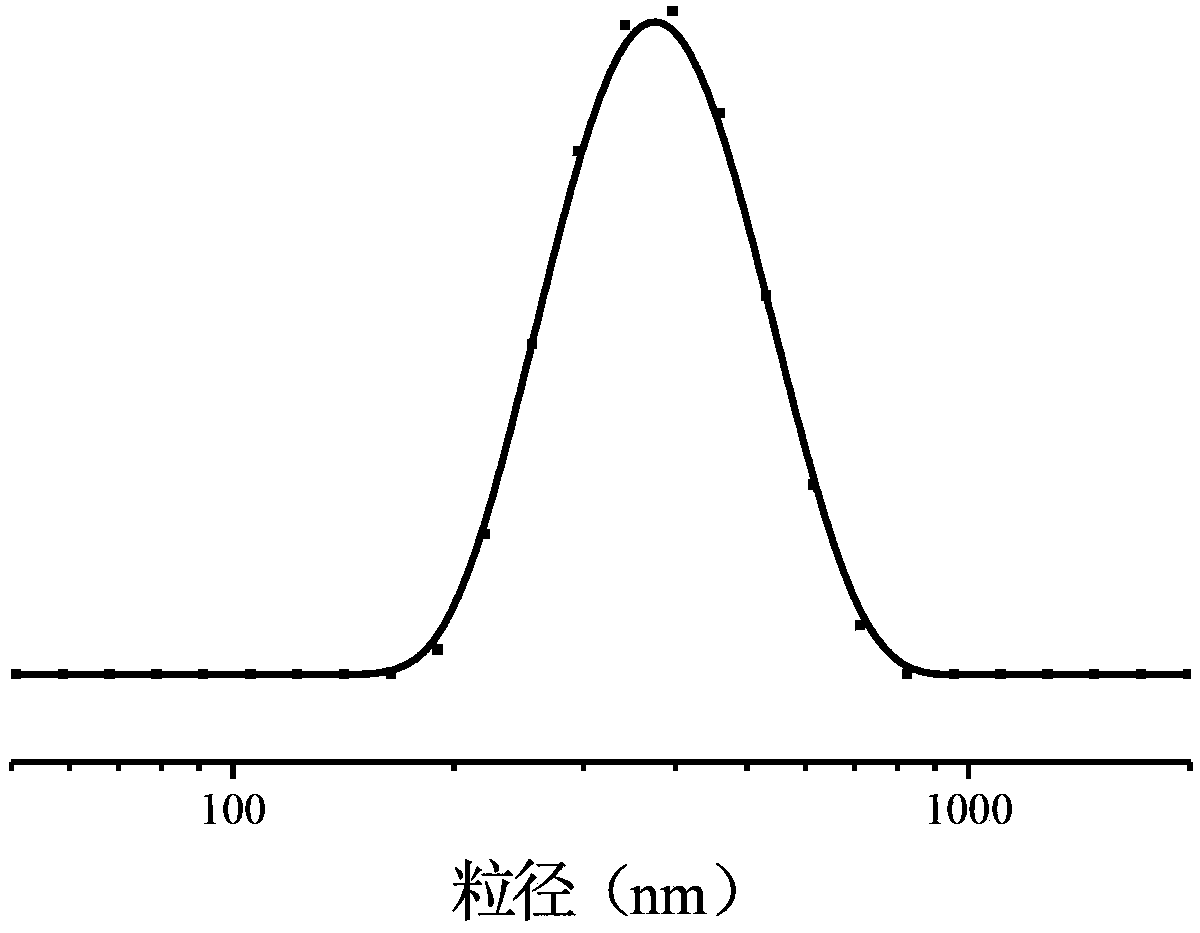
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com