Cell-penetrating peptide modified nanoparticle and its preparation method
A technology of nanoparticles and membrane-penetrating peptides, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas to increase cell uptake, bioavailability, and oral bioavailability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
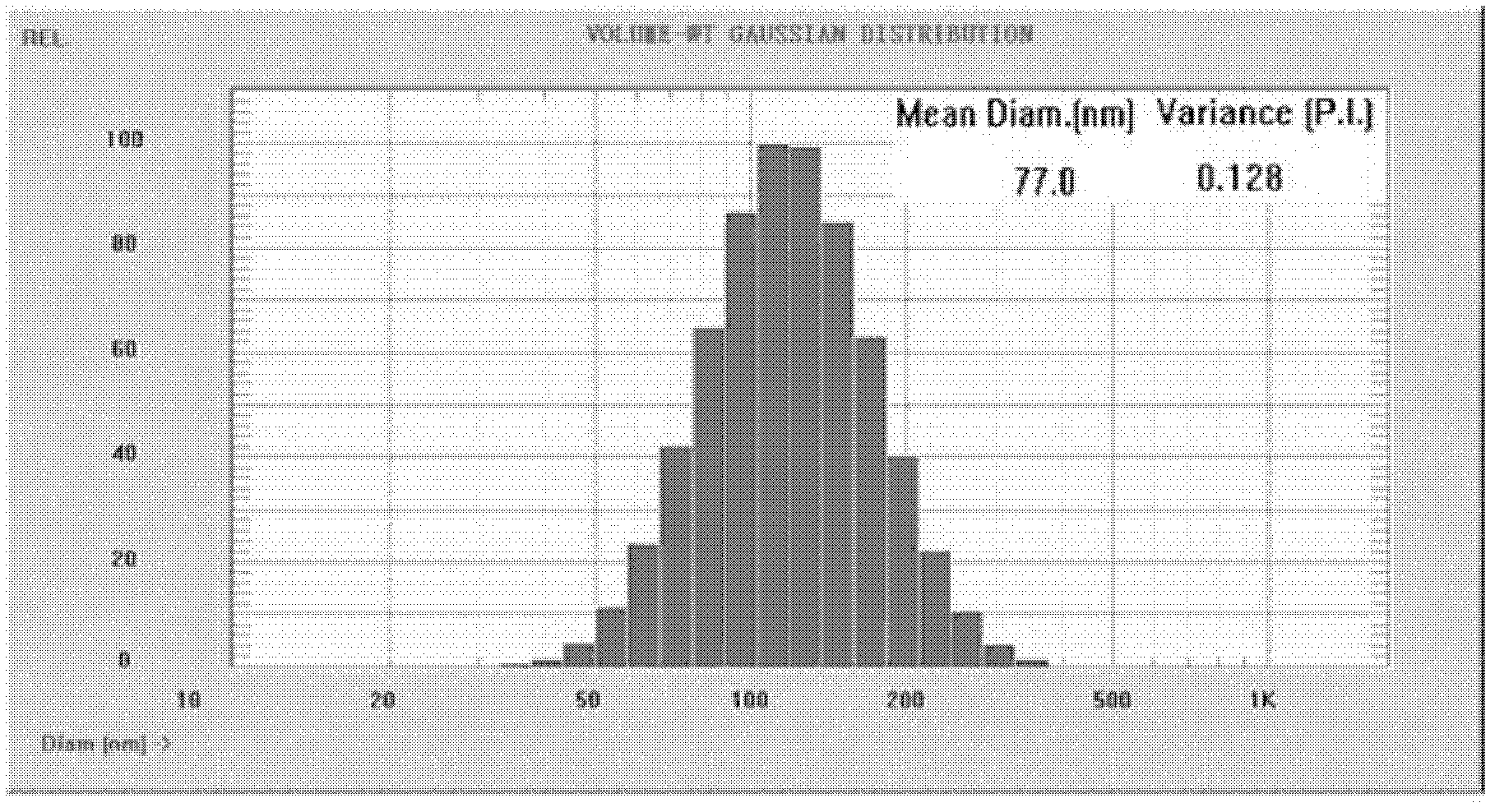
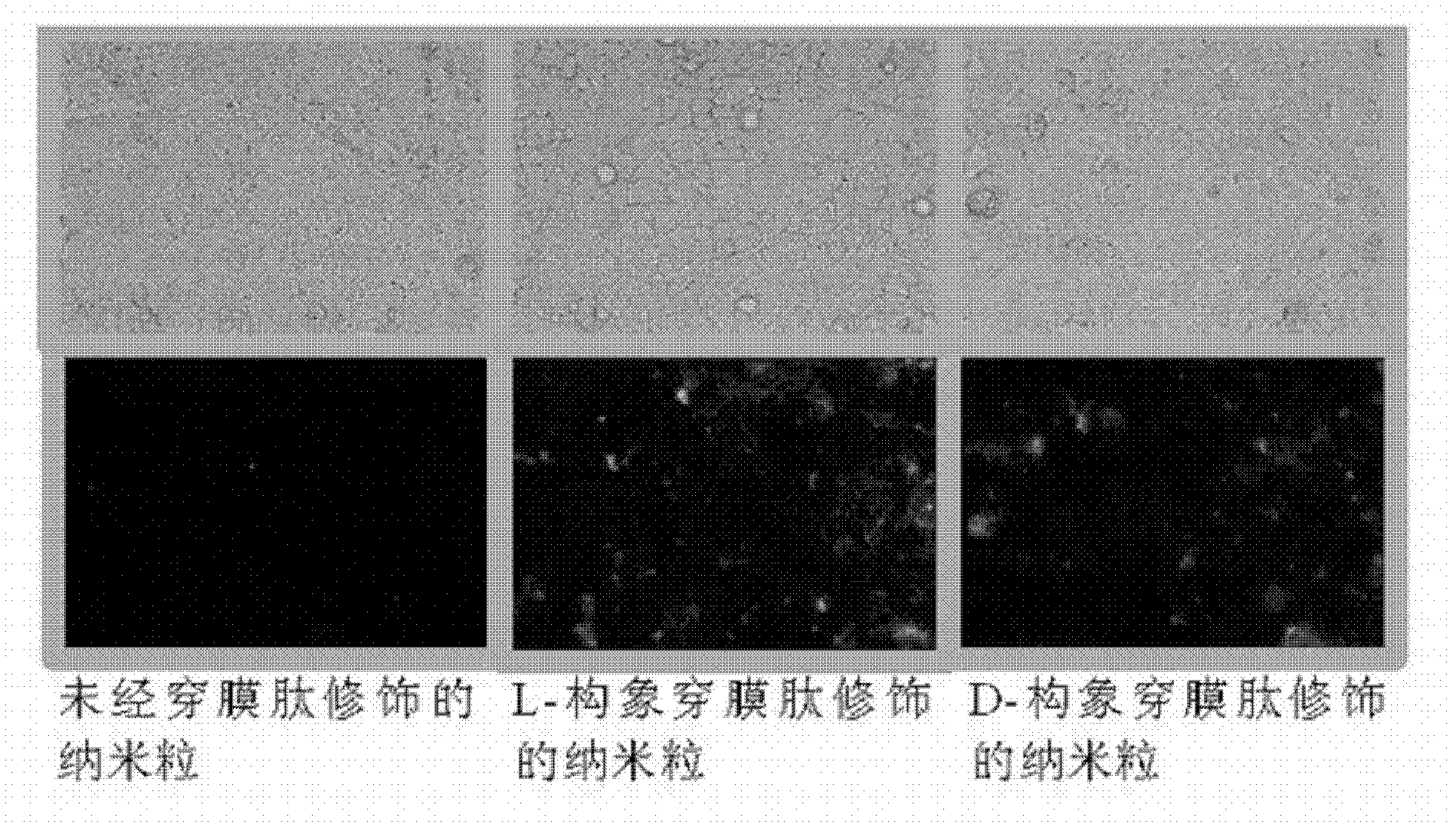
[0049] Weigh 40mg PLGA and 40mg MAL-PEG-PLGA respectively and dissolve in 5mL acetone and ethanol (8:2) mixed solvent to form the organic phase. Under continuous stirring, the organic phase was dropped into 30 mL of 0.2% polysorbate-80 (Tween80) aqueous solution. After stirring for half an hour, spin evaporate to completely volatilize the acetone-ethanol, and solidify to obtain the PLGA / MAL-PEG-PLGA nanoparticle dispersion system. Add an appropriate amount of Poly(Arg) to the neutral nanoparticle solution n -Cys(n=4-20) solution, stirred for half an hour, dialyzed to remove free Poly(Arg) n -Cys get the required Poly(Arg) n modified nanoparticles.
Embodiment 2
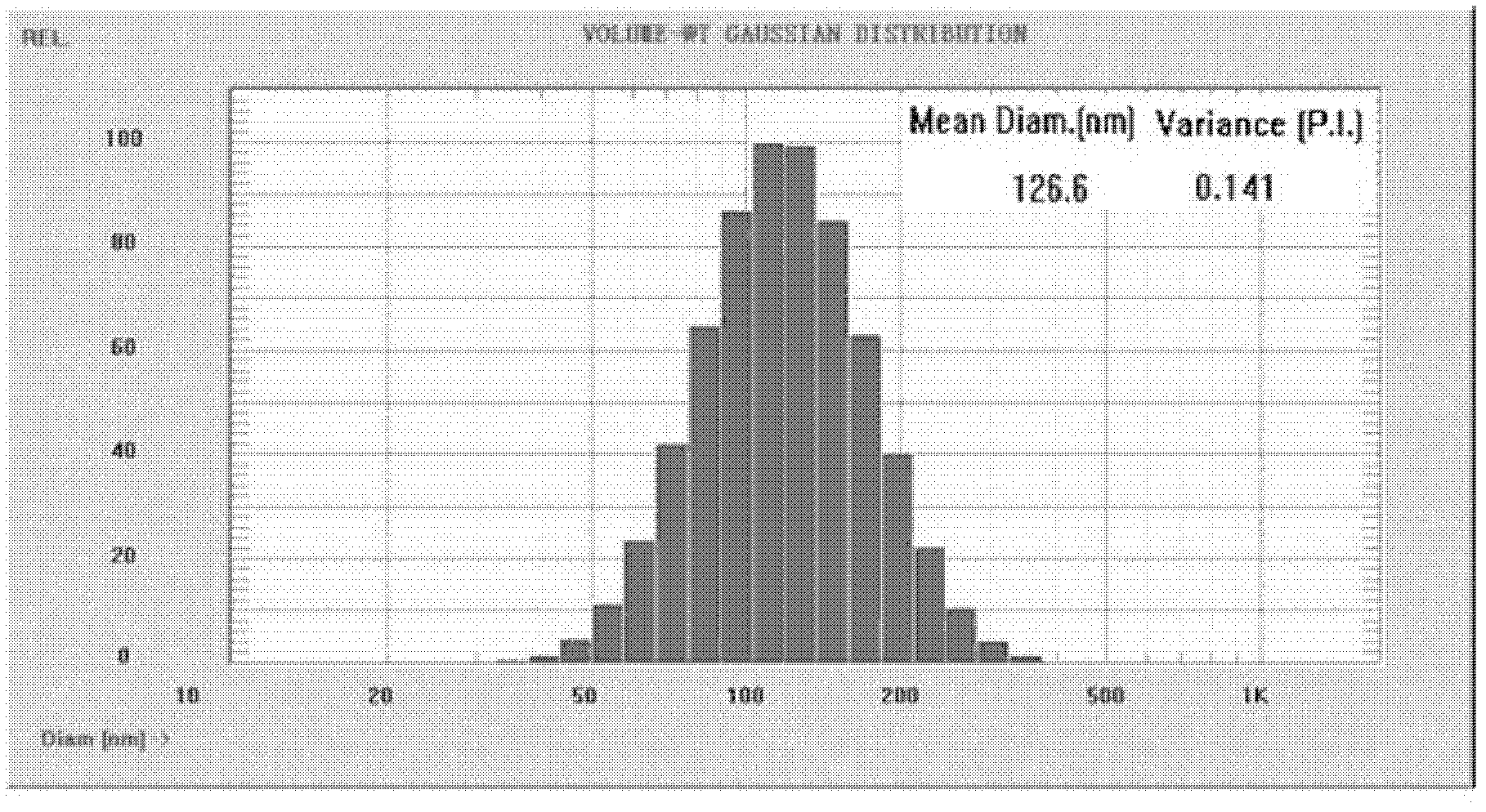
[0051] Weigh PLGA and MAL-PEG-PLGA at a mass ratio of 90:10, dissolve them in 1.0 mL of dichloromethane, add 50 μL of 7.5 mg / mL insulin aqueous solution, perform ultrasonic emulsification for 30 seconds, and then add 0.5% sodium cholate aqueous solution 2 mL, intermittent ultrasonic emulsification for 30 s, the prepared double emulsion was transferred to 20 mL of aqueous solution containing 0.5% sodium cholate, rotary evaporation for 20 min to remove the organic solvent, 25000 g / min low temperature centrifugation for 30 min to obtain the desired nanoparticles. Redisperse nanoparticles with Hepes buffer salt, add appropriate amount of Poly(Arg) n -Cys(n=4-12) solution, stirred for half an hour, dialyzed to remove free Poly(Arg) n -Cys, get the desired surface modification Poly(Arg) nAnd nanoparticles loaded with insulin. After testing, the drug loading capacity of the nanoparticles is 2.1% (w / w).
Embodiment 3
[0053] Weigh PLGA and MAL-PEG-PLGA at a mass ratio of 80:20, dissolve them in 1.0 mL of dichloromethane, add 50 μL of 2 mg / mL leuprolide aqueous solution, perform ultrasonic emulsification for 30 seconds intermittently, and then add 0.5% cholic acid Sodium aqueous solution 2mL, intermittent ultrasonic emulsification for 30s, the prepared double emulsion was transferred to 20mL aqueous solution containing 0.5% sodium cholate, rotary evaporation for 20min to remove the organic solvent, 25000g / min low temperature centrifugation for 30min to obtain the desired nanoparticles. Redisperse nanoparticles with Hepes buffer salt, add appropriate amount of Poly(Arg) n -Cys(n=6-10) solution, stirred for half an hour, dialyzed to remove free Poly(Arg) n -Cys, get the desired surface modification Poly(Arg) n And the nanoparticles containing leuprolide. After testing, the drug loading capacity of the nanoparticles is 1.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com