Preparation method of manidipine sustained release tablet
A sustained-release tablet, gentle technology, applied in the field of manidipine sustained-release tablets, to achieve the effect of simple preparation process and small gastrointestinal side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The formulation of the plain tablets of manidipine sustained-release tablets is as follows: 500 mg of manidipine, 200 mg of carrageenan, 50 mg of magnesium stearate, 100 mg of citric acid, and 200 mg of lactose; the coating formulation of manidipine sustained-release tablets is as follows: carboxymethyl Sodium cellulose base 10.5mg, propylene glycol 20mg, talcum powder 9.5mg.
[0022] Preparation Process:
[0023] (1) Dissolving 100mg of carrageenan in an ethanol solution to prepare an alcohol solution; (2) Place the sieved manidipine, remaining carrageenan, citric acid, and lactose in a high-efficiency mixing granulator to fully mix , add the alcohol solution of the above-mentioned carrageenan, and mix well to obtain a soft material; (3) dry the above-mentioned soft material and add a lubricating glidant to obtain a mixed powder, which is all added to a multi-directional motion mixer, using a rotary Tablet machine tableting to obtain plain tablets; (4) preparation of ...
Embodiment 2
[0025] The formulation of the plain tablets of manidipine sustained-release tablets is as follows: 500 mg of manidipine, 300 mg of carrageenan, 20 mg of magnesium stearate, 50 mg of citric acid, and 100 mg of lactose; the coating formula of manidipine sustained-release tablets is as follows: carboxymethyl Cellulose Sodium 9.5mg, Propylene Glycol 19mg, Talc 15mg.
[0026] Preparation Process:
[0027] (1) Dissolving 100mg of carrageenan in an ethanol solution to prepare an alcohol solution; (2) Place the sieved manidipine, remaining carrageenan, citric acid, and lactose in a high-efficiency mixing granulator to fully mix , add the alcohol solution of the above-mentioned carrageenan, and mix well to obtain a soft material; (3) dry the above-mentioned soft material and add a lubricating glidant to obtain a mixed powder, which is all added to a multi-directional motion mixer, using a rotary Tablet machine tableting to obtain plain tablets; (4) preparation of coating solution: tal...
Embodiment 3
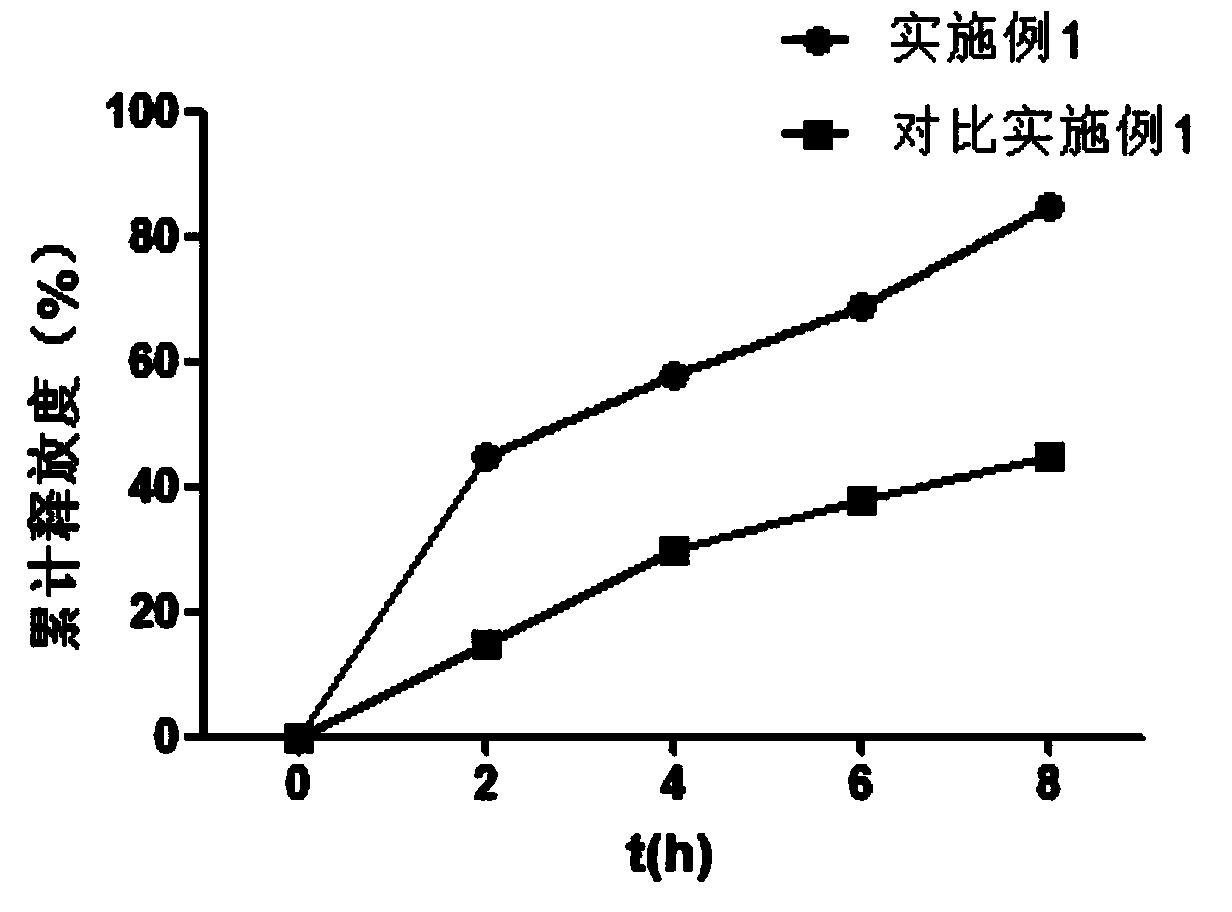
[0028] The release research of embodiment 3 manidipine sustained-release tablets
[0029] Tablets prepared by direct compression of the tablet (specification 50 mg) prepared in Example 1 of the present invention and manidipine (see figure 1 In Comparative Example 1) for comparison.
[0030] Release test method: Take the tablet (50 mg specification) prepared in Example 1 and the tablet (50 mg specification) prepared by direct compression of manidipine respectively, according to the release test method (Chinese Pharmacopoeia 2010 edition two appendix XD first method), use 900ml of distilled water as solvent, rotate at 50 rpm, operate according to law, after 1, 4, and 8 hours, take 10ml of the solution, filter it through a 0.8um filter immediately, and add 10ml of solvent in time, and accurately measure the filtrate Appropriate amount, quantitatively diluted with distilled water to a solution containing about 16ug per 1ml, according to the spectrophotometric method (Chinese Phar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com