Resveratrol and temozolomide double-medicine-loaded nanospheres, as well as application and preparation method thereof
A technology of resveratrol and temozolomide is applied in the field of anti-glioma double-drug nano-drug-loaded microspheres and the preparation thereof, which can solve the problems of affecting a wide range of applications, insufficient clinical efficacy, large oral dose and the like, and achieves the preparation method. Simple and easy, superior anti-tumor efficacy, enhanced synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
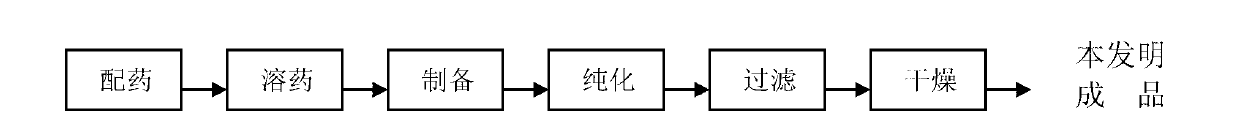
[0046] Step 1, dispensing: Weigh 30g of temozolomide (TEM), 30g of resveratrol (RES) and 940g of drug-loaded materials according to the weight ratio of the raw material components, wherein the drug-loaded materials are polycaprolactone and polyethylene glycol Amphiphilic block copolymer polycaprolactone-polyethylene glycol (mPEG-PCL) synthesized by diol, in which: mPEG molecular weight is 4000, PCL molecular weight is 20000 (molecular weight is mainly distributed in this interval);
[0047] Step 2, drug dissolving: under normal temperature conditions, dissolve the raw material components such as 30g resveratrol (RES) and 940g drug-loaded material weighed in step 1 in a certain amount of acetone solvent (according to the weight of the components) Ratio, raw material component 80, acetone solvent 20); 30g temozolomide (TEM) weighed in step 1 was dissolved in a certain amount of distilled water at 45-50 degrees Celsius, (wherein according to the weight ratio of the components, tem...
Embodiment 2
[0054] Step 1, dispensing: Weigh 100g of temozolomide (TEM), 100g of resveratrol (RES) and 800g of drug-loaded materials according to the weight ratio of the raw material components, wherein the drug-loaded materials are polycaprolactone and polyethylene glycol Amphiphilic block copolymer polycaprolactone-polyethylene glycol (mPEG-PCL) synthesized by diol, in which: mPEG molecular weight is 4000, PLA molecular weight is 40000;
[0055] Step 2, drug dissolving: at room temperature, dissolve the raw material components such as 100g resveratrol (RES) and 800g drug-loaded material weighed in step 1 in a certain amount of ethanol solvent (according to the weight of the components) Ratio, raw material component 60, acetone solvent 40); Dissolve the 100g temozolomide (TEM) weighed in step 1 in a certain amount of distilled water at 45-50 degrees Celsius, wherein according to the weight ratio of the components, temozolomide 40, distilled water 60;
[0056] Step 3, preparation: add th...
Embodiment 3
[0062]Step 1, dispensing: Weigh 150g temozolomide (TEM), 150g resveratrol (RES) and 700g drug-loaded material according to the weight ratio of the raw material components, wherein the drug-loaded material is polycaprolactone and polyethylene glycol Amphiphilic block copolymer polycaprolactone-polyethylene glycol (mPEG-PCL) synthesized by diol, in which: mPEG molecular weight is 4000, PLGA molecular weight is 36000;
[0063] Step 2, drug dissolving: at room temperature, dissolve the raw material components such as 150g resveratrol (RES) and 700g drug-loaded material weighed in step 1 in a certain amount of acetone solvent (according to the weight of the components) Ratio, raw material component 50, acetone solvent 50); Dissolve 100g temozolomide (TEM) weighed in step 1 in a certain amount of distilled water at 45-50 degrees Celsius, wherein according to the weight ratio of the components, temozolomide 50, distilled water 50;
[0064] Step 3, preparation: add the resveratrol ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com