Aqueous drug preparation of anti-TNF (tumor necrosis factor)-alpha human monoclonal antibody for strengthening stability
A technology for human monoclonal antibodies and pharmaceutical preparations, which is applied in the fields of antibodies, drug combinations, and drug delivery. It can solve the problems that mannitol cannot play a stabilizing effect, the finished product should not be placed for a longer period of time, and affect the stability of antibodies.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1. Preparation of buffer solution
[0051] 1.1 Prepare 8L disodium hydrogen phosphate / citric acid monohydrate ultrafiltration buffer solution (ultrafiltration buffer solution A)
[0052] Weigh 16g of disodium hydrogen phosphate, 12.32g of citric acid monohydrate, 120g of sorbitol, and 49.28g of sodium chloride, and add them to 7.9L with water for injection. After completely dissolving and mixing, adjust the pH to 5.2 with 1M sodium hydroxide. Set the volume to 8.0L for later use.
[0053] 1.2 Prepare 8L disodium hydrogen phosphate / sodium dihydrogen phosphate and sodium citrate / citric acid ultrafiltration buffer solution (ultrafiltration buffer solution B)
[0054] Weigh 6.88g sodium dihydrogen phosphate dihydrate, 12.24g disodium hydrogen phosphate dihydrate, 2.4g sodium citrate, 10.4g citric acid monohydrate, 96g mannitol, 49.28g sodium chloride, add water for injection to 7.9 L, after being completely dissolved and mixed evenly, adjust the pH to 5.2 with ...
Embodiment 2
[0057] Embodiment 2. antibody concentration is the preparation of the ultrafiltration concentrate of 51g / L
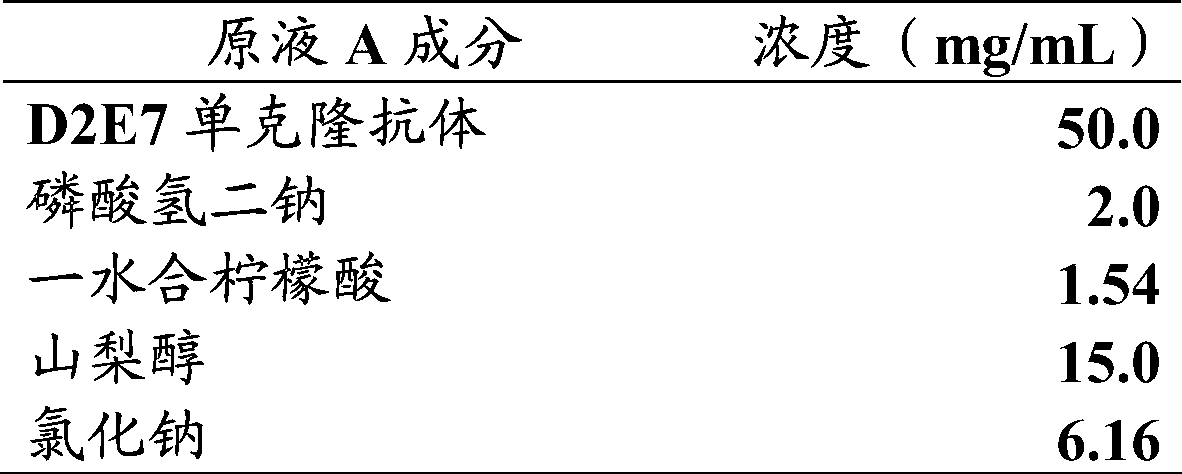
[0058] 2.1 The buffer solution is the preparation of the ultrafiltration concentrate of disodium hydrogen phosphate / citric acid monohydrate solution (ultrafiltration concentrate A)
[0059] Ultrafiltration instrument VIVA Flow 200 (manufacturer Sartorius) was cleaned with 0.2M sodium hydroxide according to the manufacturer's recommended steps, including cleaning the ultrafiltration membrane, ultrafiltration cup, pipeline, outlet, etc., and then using 0.5L of the ultrafiltration buffer solution in Example 1.1 A wash to remove sodium hydroxide. Add 0.48L of anti-TNF-α human monoclonal antibody solution (antibody concentration 25g / L, total antibody 12g) into the ultrafiltration cup, start equal volume replacement with ultrafiltration buffer solution A, the replacement volume is 4.8L. After changing the liquid, the antibody solution was concentrated from 0.48L to about 0.2...
Embodiment 3
[0064] Embodiment 3. the preparation of stoste
[0065] 3.1 Prepare 0.9mL / vial stock solution A (buffer solution is disodium hydrogen phosphate / citric acid monohydrate, preparation without Tween)
[0066] Under sterile conditions, take 50mL of the ultrafiltration concentrate A of Example 2.1, add 1mL of the ultrafiltration buffer solution A of Example 1.1 filtered through a sterile 0.22μm sterile filtration membrane without pyrogens, and mix well to obtain the stock solution a. Under aseptic conditions, it is divided into 2mL sterile pyrogen-free liquid injection vials, the filling volume is 0.9mL / bottle, divided into 54 tubes, and then sealed and stored at 2-8°C in the dark until use.
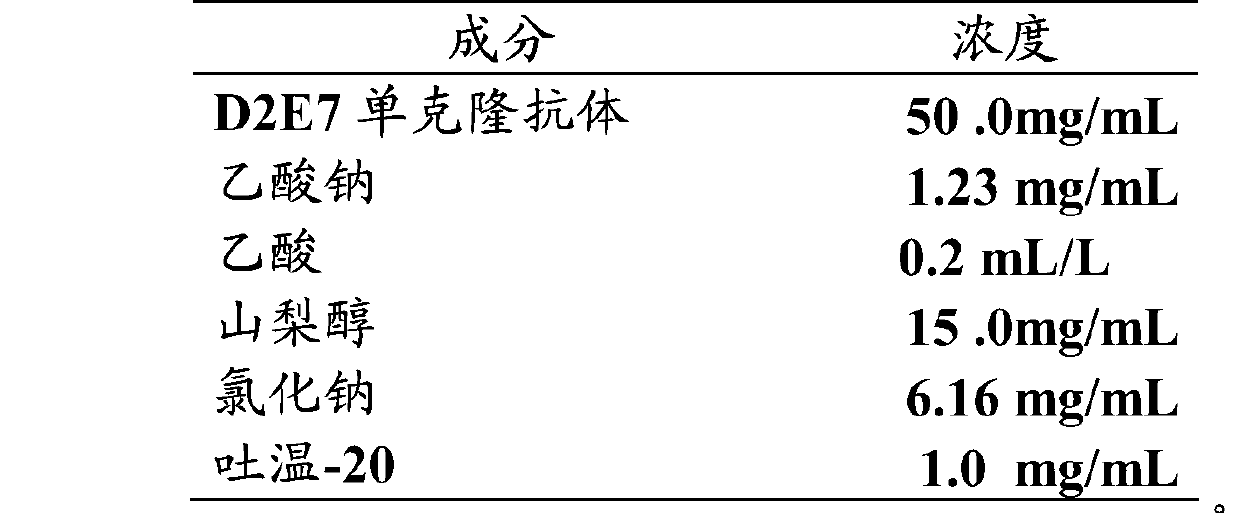
[0067] Description of each bottle: each bottle contains 0.9mL, and the labeled volume is 0.8mL. Its composition is as follows:
[0068]
[0069] 3.2 Prepare 0.9mL / vial stock solution B (buffer solution is disodium hydrogen phosphate / sodium dihydrogen phosphate and sodium citrate / citric ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com