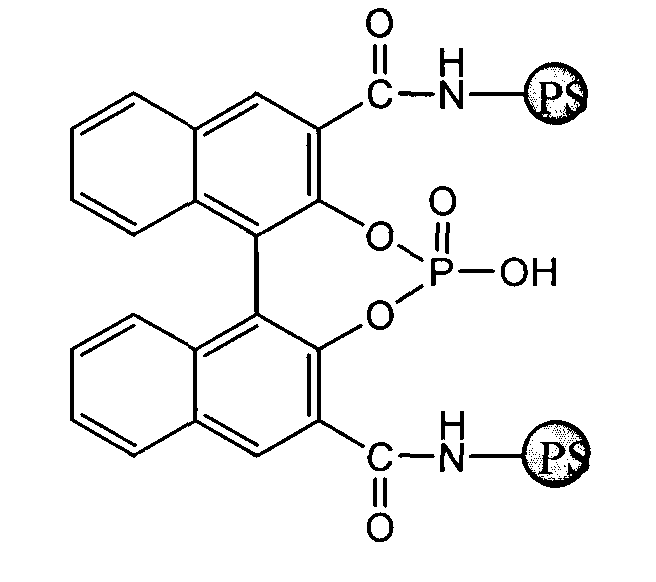
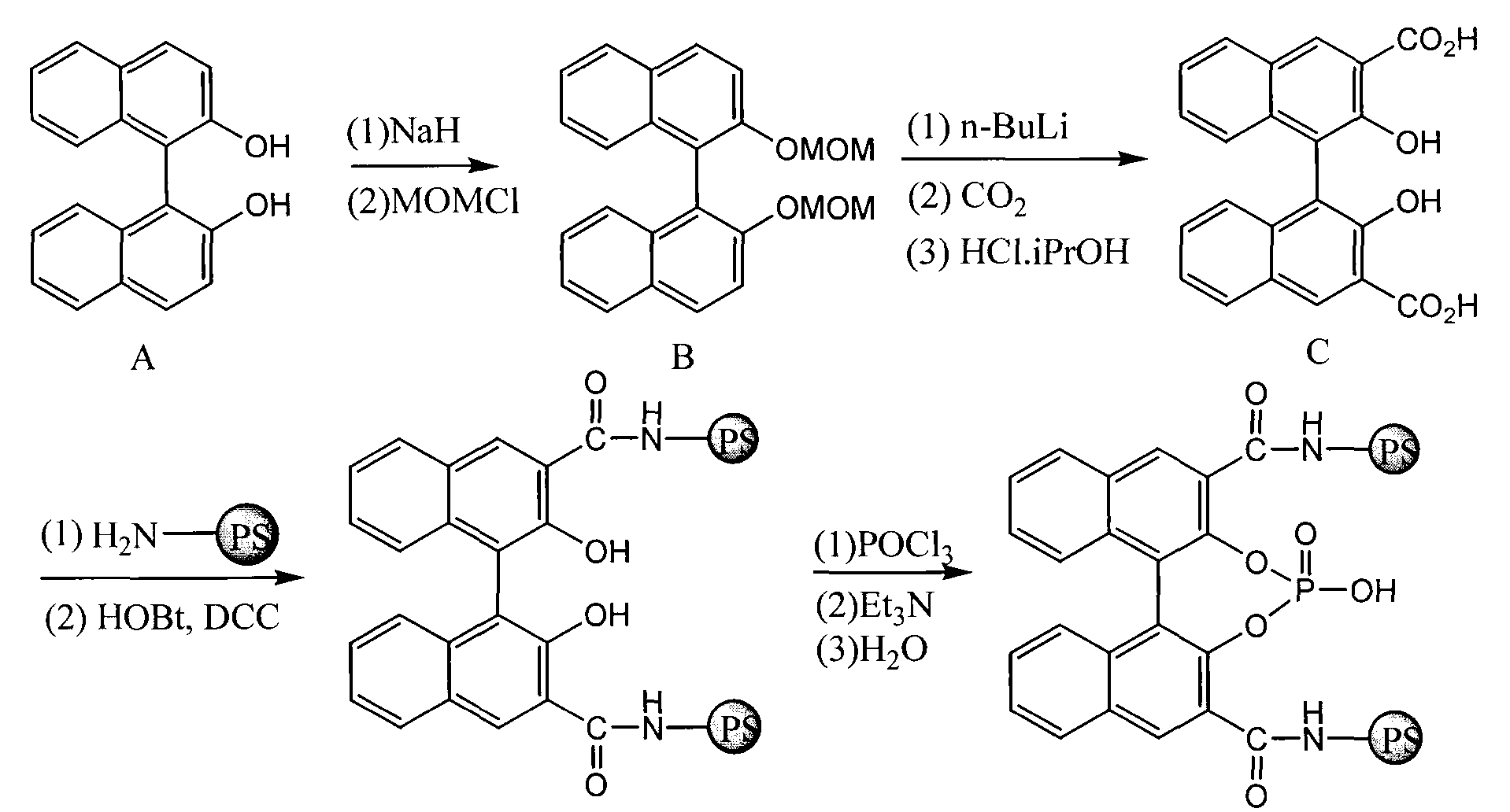
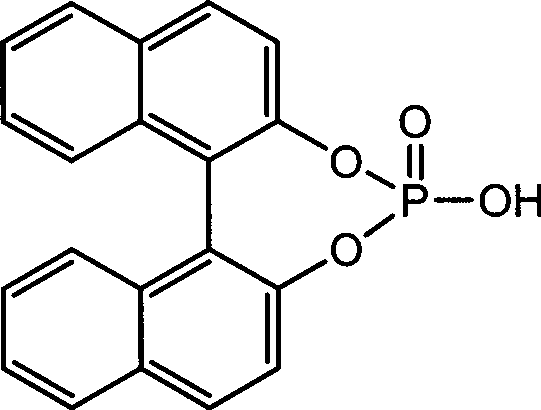
Immobilized chiral phosphoric acid catalyst and preparation method thereof
A phosphoric acid catalyst, chiral technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. Easy to purify, easy to prepare, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthesis of the chiral binaphthol (formula II) that the polystyrene resin containing amino group of embodiment 1 is immobilized
[0023] Obtained by reference method (Xiao-Wu Yang, Jian-Heng Sheng, Chao-Shan Da etc. J. Org. Chem., 2000, 65(2), 295-296). Infrared spectrum IR (KBr) of compound (formula II): 3344, 2827, 1644, 1626, 1570, 1308, 1270, 1245cm -1 . We have carried out infrared spectrometry respectively to compound C, polystyrene resin and resin-linked chiral binaphthol (formula II), and find out by comparison, after resin and compound C condensation, carboxyl peak 3589, 1672cm -1 , and resin amino peak 3535cm -1 disappear while at 3344, 1644, 1570, 1245cm -1 The characteristic absorption peaks of amides appear at 1308 and 1270cm -1 It shows the existence of phenolic hydroxyl group, which proves that BINOL has been bonded to the resin. The immobilized amount of chiral binaphthol on polystyrene resin was calculated to be 0.4mmol / g.
Embodiment 2
[0024] The synthesis of the chiral phosphoric acid (formula I) that the polystyrene resin of embodiment 2 is immobilized
[0025] Take a 50mL dry three-necked bottle, under the protection of argon, add 6 grams of polystyrene resin immobilized chiral binaphthol (formula II) and 20mL CH 2 Cl 2 , cooled to 0°C, followed by dropwise addition of POCl 3 (3.6mL, 39.2mmol), Et 3 N (8.2 mL, 58.8 mmol), after the dropwise addition, stirred at room temperature for 4-6 hours. Cool the system to 0°C, slowly add water (1mL) dropwise, filter the solid with suction, wash with DMF, CH 2 Cl 2 , 1N HCl aqueous solution and methanol wash 2 times, vacuum-dry to constant weight, obtain the chiral phosphoric acid (formula I) of target product polystyrene resin immobilization, quality is 6.15 grams, calculate chiral phosphoric acid on polystyrene resin The solid loading capacity is 0.4mmol / g.
[0026] Infrared spectrum IR (KBr) of compound (formula I): 3397, 3344, 2827, 1735, 1644, 1626, 1570, ...
Embodiment 3
[0028] Example 3 (Comparative Example) Unsupported chiral phosphoric acid (formula III) catalyzes the asymmetric Friedel-Crafts reaction of indole and sulfonimide
[0029]
[0030] Take a 50mL dry three-necked bottle, under argon protection, dissolve imine E (2.5mmol) and unsupported chiral phosphoric acid catalyst (formula III) (0.25mmol) in toluene (10mL), and stir at room temperature for 30 minutes, then cooled to -50°C and stirred for 10 minutes, and added indole D (12.5mmol) at -50°C. After TLC tracking imine E raw material disappeared, add 10% NaHCO 3 The reaction was quenched with aqueous solution (30mL), extracted with ethyl acetate (50mL), the organic layer was washed with water (30mL) and saturated brine (30mL) respectively, and anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure, and column chromatography (AcOEt:PE=1:3) gave the target product F as a white solid with a yield of 81% and an ee value equal to zero. 1 H NMR (300MHz, CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com