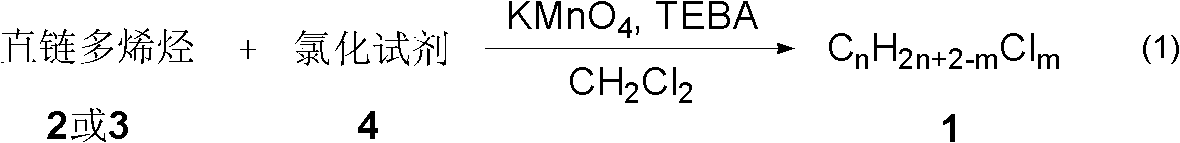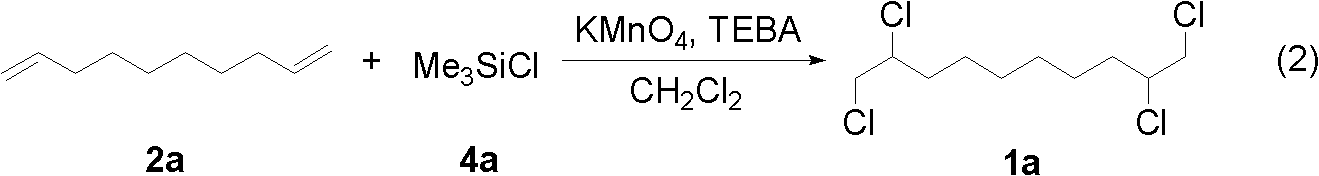Synthesizing method of polychloroalkane
A technology for polychlorinated alkanes and synthesis methods, applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve the problems of difficult separation and purification of SCCPs monomer compounds, and achieve easy separation and purification, simple steps, and applicable scope. wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] In a 50 mL reaction flask, benzyltriethylammonium chloride (0.328 g, 1.44 mmol), 20 mL of dichloromethane and potassium permanganate (0.228 g, 1.44 mmol) were sequentially added, and the reaction was stirred at room temperature for 45 minutes. After cooling to 0°C in an ice-water bath, trimethylchlorosilane 4a (0.626 g, 5.76 mmol) was added dropwise to the solution. After the dropwise addition was completed, the mixture was kept stirring at 0°C for 30 minutes, then 1,9-decadiene 2a (0.100 g, 0.72 mmol) was added, and the reaction was allowed to rise to room temperature for 45 hours. Stop the reaction, pour the reaction solution into 40 mL of 0.4M aqueous sodium thiosulfate solution to quench the reaction, and separate the organic phase. The aqueous phase was extracted with dichloromethane (3×20 mL), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the volatile components were removed under reduced pressure, and then separa...
Embodiment 2
[0040]
[0041] The reaction steps and operations are the same as in Example 1, except that the chlorination reagent is oxalyl chloride 4b, benzyltriethylammonium chloride and potassium permanganate react at room temperature for 45 minutes and then cool to -40°C The addition reaction was started, and the post-treatment gave the target product 1a (0.159 g, yield 78%) as a colorless liquid. The target product 1a was confirmed by NMR characterization and gas chromatography-mass spectrometry (GC / MS) determination.
Embodiment 3
[0043]
[0044] In a 250mL reaction flask, benzyltriethylammonium chloride (4.988g, 21.90mmol), 120mL of dichloromethane and potassium permanganate (3.461g, 21.90mmol) were successively added, and the reaction was stirred at room temperature for 45 minutes. After cooling to 0° C. in an ice-water bath, trimethylchlorosilane 4a (9.517 g, 87.60 mmol) was added dropwise. After the dropwise addition was completed, the reaction was continued at 0°C for 30 minutes, then 1,5,9-decatriene 2b (1.000 g, 7.30 mmol) was added, and the mixture was naturally raised to room temperature for 45 minutes. Stop the reaction, pour the reaction solution into 240mL 0.4M sodium thiosulfate solution to quench the reaction, and separate the organic phase. The aqueous phase was extracted with dichloromethane (3×60mL), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the volatile components were removed under reduced pressure, and then separated by silica gel column ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com