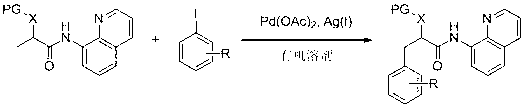Method for synthesizing substituted phenylalanine and substituted phenyllactic acid derivatives by using palladium as catalyst
A technology of phenylalanine and phenyllactic acid, which is applied in the production of bulk chemicals and organic chemistry, can solve the problems of difficult activation, and achieve the effects of high selectivity, mild reaction conditions and strong reaction versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
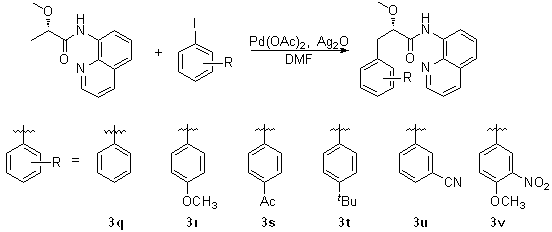
[0025] In the reactor, add 1 mmol of L-alanine whose nitrogen end is protected with a phthaloyl group and whose carboxyl end is introduced into 8-aminoquinoline, 1.2 mmol of 4-methoxy-3-nitroiodobenzene 2a , 0.1 mmol palladium acetate catalyst, 1.5 mmol silver tetrafluoroborate and 10 ml tert-butanol, at 75 o After C reacted for 24 hours, the reaction was terminated for post-treatment, and the monoarylation product such as structural formula 1 was obtained by silica gel column chromatography or dichloromethane / petroleum ether mixed solvent beating 3a The yield was 79%, and the yield of the bisarylated product of structural formula 2 was 3%. 1 H NMR (400 MHz, CDCl 3 ) δ 10.28 (s, 1 H), 8.78 – 8.66 (m, 1 H), 8.60 (d, J = 2.7 Hz, 1 H), 8.13 (dd, J = 8.3, 1.4 Hz, 1 H), 7.86 (dt, J = 7.1, 3.5 Hz, 2 H), 7.75 (dd, J = 5.5, 3.1 Hz, 2 H), 7.57 – 7.47 (m, 3 H), 7.40 (dd, J = 8.2, 4.3 Hz, 1 H), 6.98 (d, J = 8.7 Hz, 1 H), 5.38 (dd, J = 10.3, 6.2 Hz, 1H), 3.89 (s, 2H), 3...
Embodiment 2~16
[0029] The operation steps are the same as in Example 1, the difference is that by changing the substituent R of the aryl iodide, different β-monoarylalanine derivatives can be obtained (see Table 1).
[0030]
[0031] Table 1: Experimental results of monoarylation of alanine derivatives
[0032] Numbering R 3 Yield b (%) 1 4-OMe-3-NO 2 ( 2a ) 3a 79 2 4-Me ( 2b ) 3b 86 3 3-Me ( 2c ) 3c 87 4 4-OMe ( 2d ) 3d 82 5 3-OMe ( 2e ) 3e 82 6 2-OMe ( 2f ) 3f 71 7 4-Et ( 2g ) 3g 88 8 4-NHAc ( 2h ) 3h 79 9 4-NO 2 ( 2i ) 3i 78 10 4-Ac ( 2j ) 3j 80 11 4-F ( 2k ) 3k 62 12 4-CN ( 2l ) 3l 69 13 3,4-Me 2 ( 2m ) 3m 83 14 2,4-OMe 2 ( 2n ) 3n 85 15 3-F-4-NO 2 ( 2o ) 3o 75 16 3-NO 2 -4-OAc-5-I ( 2p ) 3p 74
[0033] Among them, the resulting product The characterization data of 3b and 3...
Embodiment 17
[0041] In the reactor, add 0.3 mmol of L-lactic acid, 0.45 mmol of 4-acetyl iodobenzene, 0.03 mmol of palladium acetate catalyst and 0.39 mmol of silver oxide and 2 ml of N, N-dimethylformamide solvent at 75 o C. After reacting for 24 hours under a nitrogen atmosphere, the reaction was terminated for post-treatment, and the monoarylation product of structural formula 3 was obtained by silica gel powder column chromatography, with a yield of 62%. 1 H NMR (400 MHz, CDCl 3 ) δ 10.73 (s, 1 H), 8.85 – 8.74 (m, 2 H), 8.14 (dd, J = 8.3, 1.5 Hz, 1 H), 7.86 (d, J = 8.2 Hz, 2 H), 7.58 – 7.50 (m, 2 H), 7.46 – 7.38 (m, 3 H), 4.09 (dd, J = 8.2, 3.6 Hz, 1 H), 3.50 (s, 3 H), 3.34 (dd, J = 14.1, 3.6 Hz, 1 H), 3.14 (dd, J = 14.1, 8.2 Hz, 1 H), 2.53 (s, 3 H).; 13 C NMR (101 MHz, CDCl 3 ) δ 197.96, 170.35, 148.68, 143.39, 138.92, 136.26, 135.68, 133.81, 129.83, 128.49, 128.05, 127.27, 122.22, 121.75, 116.70, 84.00, 77.48, 77.16, 76.84, 59.34, 39.33, 26.65.
[0042] The product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com