Water-soluble cationic polyelectrolyte with end group provided with fluorophore pyrene, and preparation method and application thereof
A water-soluble cation and polyelectrolyte technology, applied in the field of functional polymers, can solve the problems of complex preparation and limited fluorescence intensity, and achieve simple preparation, good hydrophilicity and biocompatibility, and controllable structure and performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
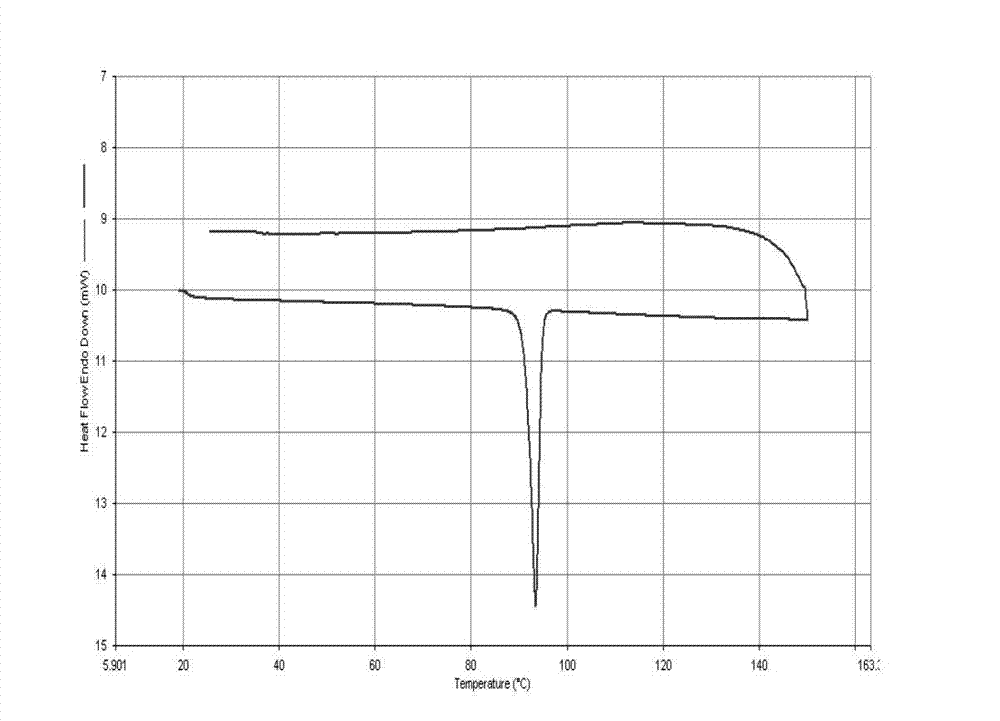
[0050] (1) The atom transfer radical polymerization initiator used is synthesized by pyrenemethanol and 2-bromo-isobutyryl bromide, and its synthetic route is shown in the following formula:
[0051]
[0052] The specific synthesis method of the initiator is as follows: Weigh 0.500g (0.0021mmol) of pyrenemethanol, 5ml of dry tetrahydrofuran, and 0.5ml (0.0038mmol) of triethylamine in a 50ml round-bottomed flask, add magnetons, and place in an ice-water bath. Measure 2ml of tetrahydrofuran, 0.5ml (0.0039mmol) of 2-bromo-isobutyryl bromide in the dropping funnel, slowly drop it into the round bottom flask; and stir at room temperature for 10 hours. After the reaction was completed, the insoluble matter bromide was filtered off, and most of the tetrahydrofuran solution was removed by rotary evaporation. The remaining material was diluted with dichloromethane, at which point the solution was pale yellow. Extract with 150ml of saturated potassium carbonate solution three times,...
Embodiment 2
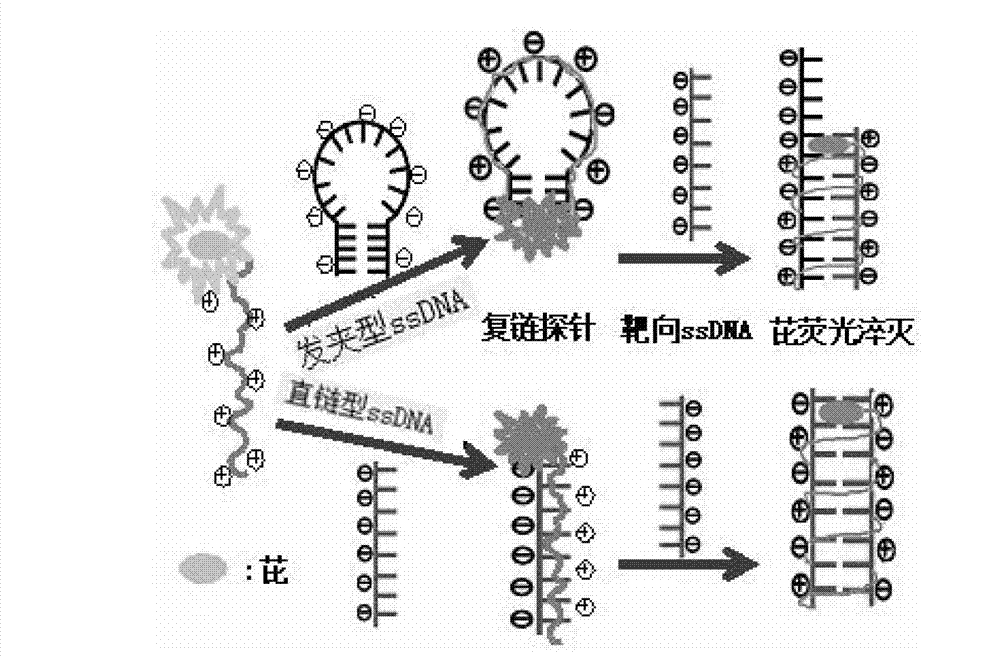
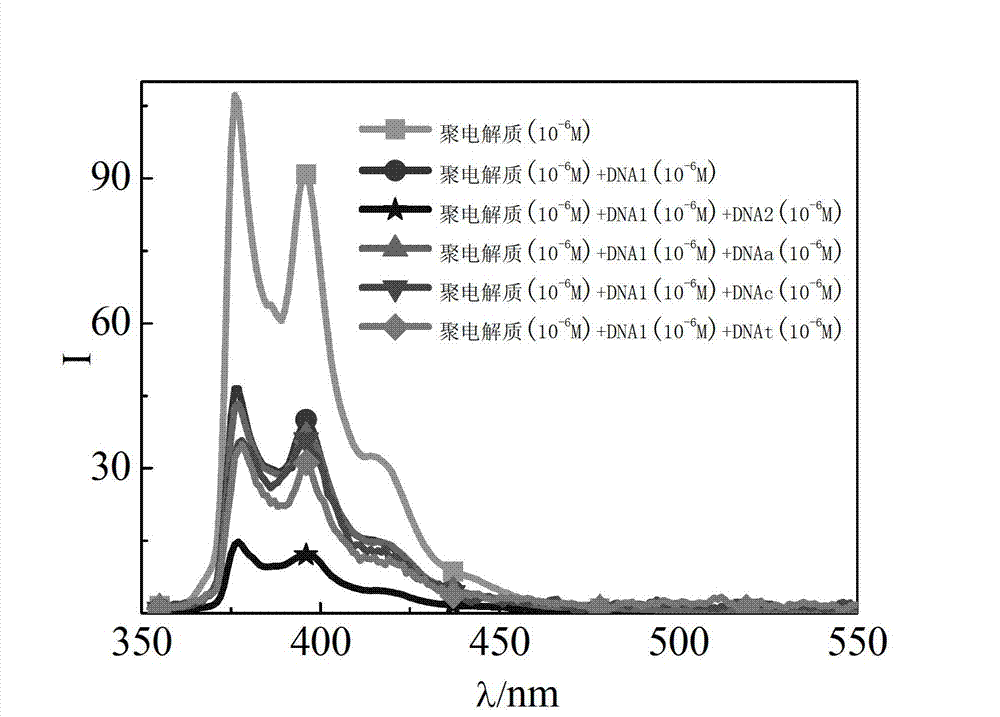
[0060] Example 2: Application of a water-soluble cationic polyelectrolyte whose end group is a fluorescent group pyrene as a polyelectrolyte fluorescent probe in the specific recognition of nucleic acid molecular base sequence structure.
[0061] (1) The water-soluble cationic polyelectrolyte whose end group is the fluorescent group pyrene is configured into 10 with phosphate buffer solution. -4 mol / L dilute solution.
[0062] (2) Different kinds of DNA primers stored in freezer were centrifuged and dissolved in an appropriate amount of phosphate buffer solution to prepare DNA dilute solution. Described DNA primer kind and base sequence are as follows:
[0063] DNA1—hairpin DNA (GCA CAA ACA AGT AGA ATG TAT GTG C);
[0064] DNA2—linear DNA complementary to DNA 1 (GCA CAT ACA TTC TAC TTG);
[0065] DNA3—linear DNA complementary to DNA 2 (GCA CAT ACA TTC TAC TTG);
[0066] DNAa—straight-chain DNA whose bases are all A (AAA AAA AAA AAA AAA AAA);
[0067] DNAc—straight-chain D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com