Kit for early diagnosis of bladder cancer and preparation method of kit
A technology for bladder cancer and urine, which is applied in the field of urine early diagnosis of bladder cancer immunoassay kit and its preparation, which can solve problems such as false positives, and achieve the effects of simple operation, rapid detection, and wide use range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of the chemiluminescent immunoassay kit for the human urinary bladder cancer tumor marker AG-α3β1 of the present invention
[0046] 1. Preparation of standard products
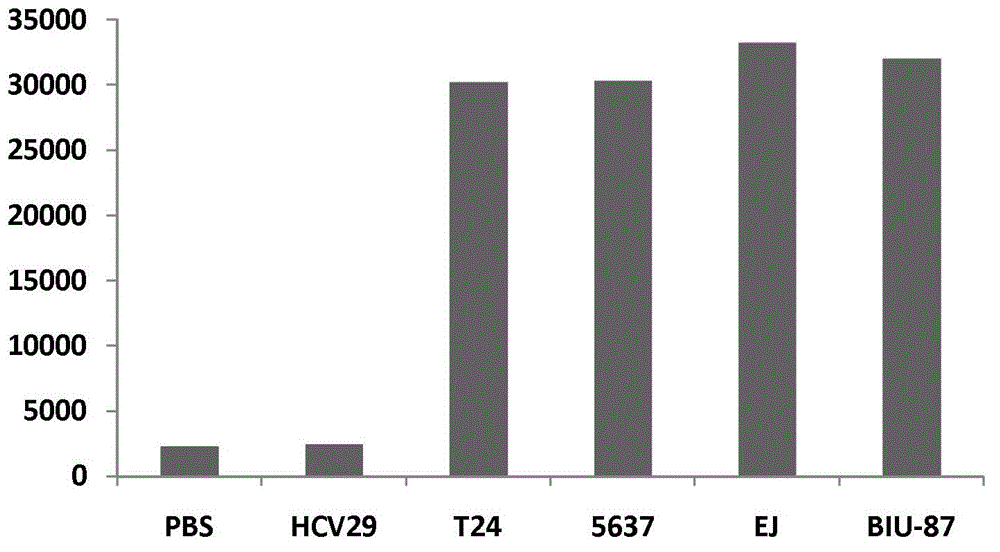
[0047] This method uses horseradish peroxidase to catalyze the luminol and hydrogen peroxide chemiluminescence system, and uses a chemiluminescence instrument to detect the amount definition of AG-α3β1 contained in the sample with a luminescence value of 6685 (that is, the Cut off value selected by this method) 1U / mL. This luminescence value is also equal to the chemiluminescence value of a human bladder cancer cell line (EJ cell) at a concentration of 1000 cells / mL.
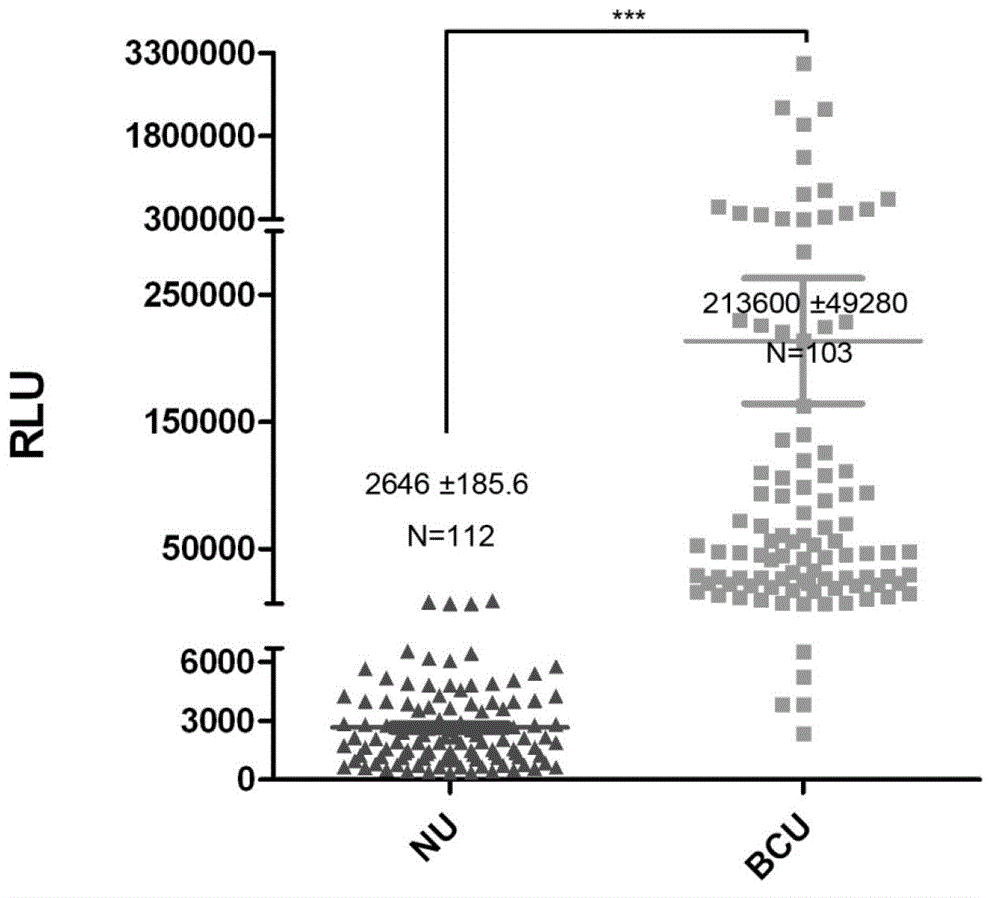
[0048] Collect a large number of urine samples from bladder cancer patients with high chemiluminescence values and normal human urine samples (the normal human urine does not contain AG-α3β1), and use normal human urine samples to gradually dilute the bladder cancer patient urine samples, The concentration gradie...
Embodiment 2
[0086] Example 2 Method of using the bladder cancer tumor marker AG-α3β1 chemical method photoimmunoassay assay kit of the present invention
[0087] 1. Sample pretreatment
[0088] Human urine is collected and analyzed directly without any other special treatment.
[0089] 2. Detection steps
[0090] Before using this kit for detection, it is necessary to take out the standard prepared in Example 1, the coated microwell plate, the enzyme-labeled monoclonal antibody solution and each buffer solution, let it stand at room temperature, and then use it after equilibrating to room temperature ; Adjust the incubator or water bath to 37°C; prepare a suitable micro-sampler and corresponding tips and check whether the chemiluminescence instrument is working normally.
[0091] The specific operation steps for using this kit are as follows:
[0092] 1) Take out the components in the kit and equilibrate to room temperature;
[0093] 2) Place the slats required for the experiment on t...
Embodiment 3
[0102] The methodological index of embodiment 3 kits of the present invention
[0103] The test kit prepared in Example 1 is tested according to the conventional manufacturing and testing procedures in the art, and the results are as follows:
[0104] 1. The standard curve of this kit
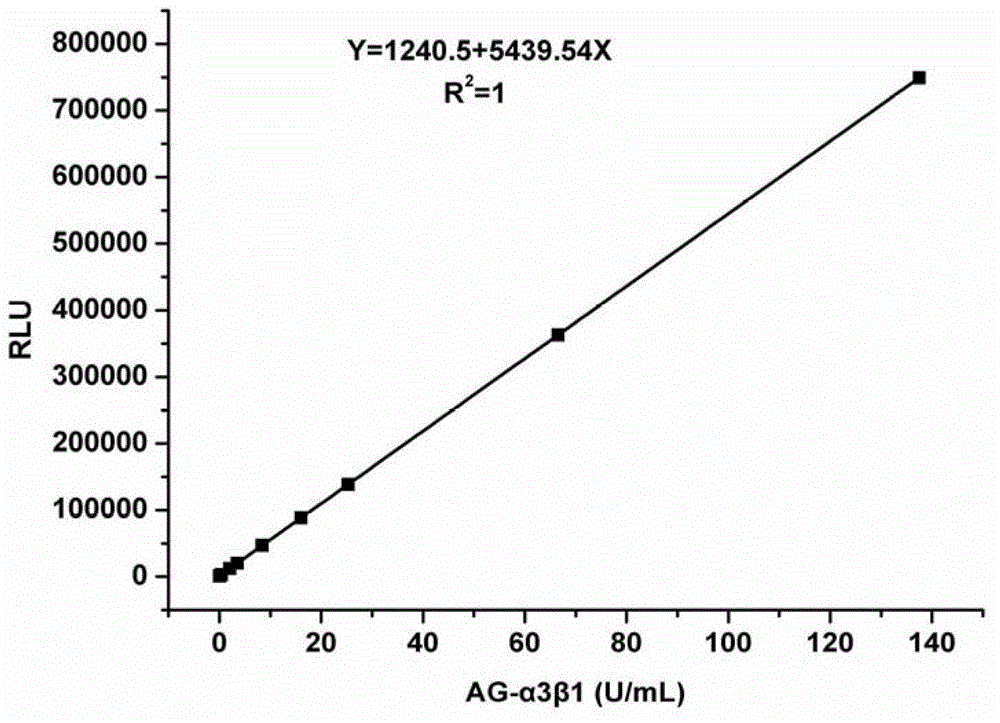
[0105] This kit uses horseradish peroxidase to catalyze the luminol and hydrogen peroxide chemiluminescence system, and uses a chemiluminescence instrument to detect the luminescence value and draw a standard curve, see the appendix figure 1 , where, I is the luminous intensity, C is the concentration of the standard cell lysate (in U / mL), and the correlation coefficient R 2 =1, Y=1240.5+5439.54X.
[0106] 2. Kit sensitivity experiment
[0107] Sensitivity is the concentration that can be distinguished from the zero dose point. Measure 10 zero standard points at the same time to obtain the average value and standard deviation of RLU. Calculate the difference between the average value of RL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com