Sulfur-containing hyperbranched epoxy resin and preparation method thereof
A technology of epoxy resin and hyperbranched polymer, which is applied in the field of sulfur-containing hyperbranched epoxy resin and its preparation, can solve the problems of long reaction time, low yield, environmental pollution and the like, and achieves short reaction time and reduced viscosity. , the effect of low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
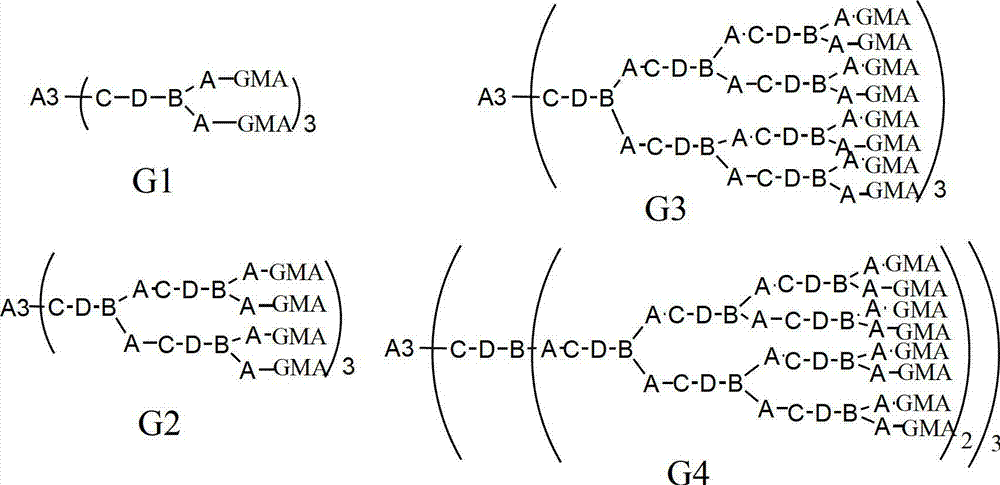
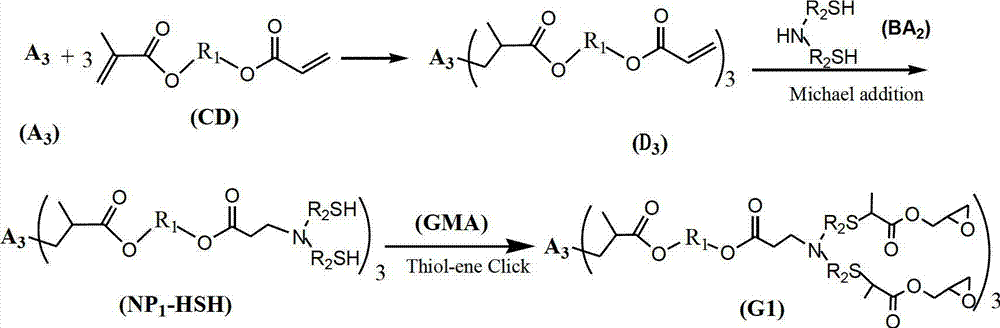
[0042] Preparation of Compound A3:
[0043]It is obtained by the esterification reaction of the corresponding trihydric alcohol with thioglycolic acid or mercaptopropionate, and the preparation of trimethylolpropane trimercaptoacetic acid ester (TMP-TEA) is taken as an example to illustrate its preparation process. Add 0.1mol trimethylolpropane, 0.45mol mercaptopropionic acid, and 1.6g p-toluenesulfonic acid to a four-neck flask equipped with a condenser, thermometer, water separator and stirrer, slowly raise the temperature to 160°C, and stir for 6 hours Or so, stop reacting. Add 20g of a saturated aqueous solution of sodium bicarbonate, then pour into a separatory funnel to separate layers, wash with 20ml of distilled water each time until the pH value is 6-7, and then rotate the oil layer at about 120°C with a vacuum of 2-3mmHg Impurities were removed to obtain 33.8 g of light yellow transparent liquid with a yield of about 95%. The remaining A3 compounds can be obtained ...
Embodiment 1
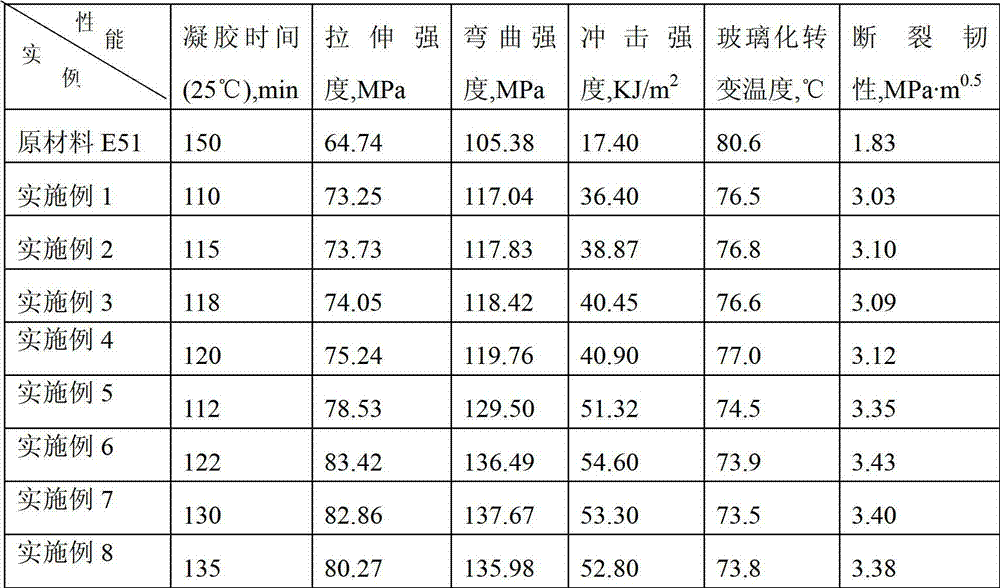
[0047] 0.1mol trimethylolpropane trimercaptoacetate (TMP-TEA) and 0.305mol hydroxyethyl acrylate-methacrylate (MA-EA-MMA) were stirred at -5-0°C for 5h, then MA was added - 0.5% hydroquinone of EA-MMA quality, and then remove excess MA-EA-MMA under the condition of 120°C vacuum degree of 2mmHg to obtain 3mol double bond-containing acrylate monomer (D3-A) ; Then add 0.31mol of dimercaptoethanolamine, stir and react at 20°C for 10h, and remove excess dimercaptoethanolamine at 120°C under a vacuum of 2mmHg to obtain the first generation of mercapto-terminated hyperbranched polymer NP1-HSH-A; Then add 0.61mol glycidyl methacrylate, stir and react at -5-0°C for 5h, then add 0.5% hydroquinone based on the mass of glycidyl methacrylate, and then keep the vacuum at 140°C under the condition of 2mmHg , to remove excess glycidyl methacrylate, to obtain the first generation of sulfur-containing hyperbranched epoxy resin (G1-A) light yellow liquid, test its number average molecular weight...
Embodiment 2
[0049] 0.1mol trimethylolpropane trimercapto propionate (TMP-TPA) and 0.32mol hydroxypropyl acrylate-methacrylate (MA-PA-MMA) were stirred at -5-0°C for 4h, then MA was added - 1.0% hydroquinone of PA-MMA quality, and then remove excess MA-PA-MMA under the condition of 120°C vacuum degree of 2mmHg to obtain 3mol double bond-containing acrylate monomer (D3-B) ; Then add 0.32mol dimercaptoisopropanolamine and stir and react at 40°C for 8h, then remove excess dimercaptoisopropanolamine at 120°C under a vacuum of 2mmHg to obtain the first generation of mercapto-terminated hyperbranched polymer NP1-HSH-B; then add 0.62mol glycidyl methacrylate, stir and react at -5-0°C for 4 hours, add 1.0% hydroquinone of GMA mass, and then add 1.0% hydroquinone at 140°C under the condition of a vacuum of 2mmHg The first generation of sulfur-containing hyperbranched epoxy resin (G1-B) light yellow liquid was obtained by removing the excess GMA. The number average molecular weight was 2300g / mol, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com