Cefixime tablets and preparation method thereof
A cefixime and tablet technology, applied in the field of cefixime tablets and their preparation, can solve the problems of affecting the speed and effect of drug treatment, slow drug absorption and distribution, low dissolution rate, etc., so as to shorten the production cycle and reduce the The effect of production cost and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
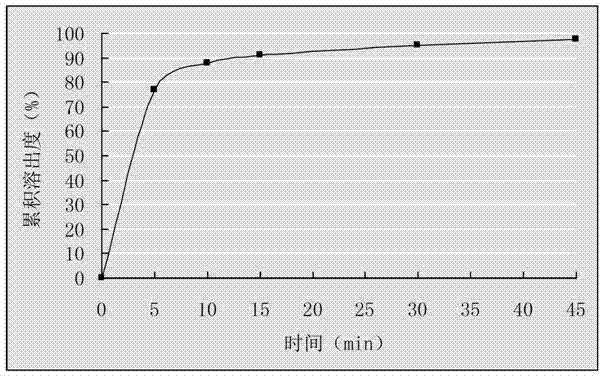
Embodiment 1
[0021] Dissolve 500g of cefixime and 750g of lecithin in 10L of methanol, add 200g of silicon dioxide, and stir; evaporate the methanol to dryness under reduced pressure below 50°C, take out the evaporated compound, grind it through a 80-100 mesh sieve, and obtain Cefixime phospholipid complex of silicon dioxide; weigh 145g of cefixime phospholipid complex containing silicon dioxide, mix with 55g microcrystalline cellulose, 45g lactose, 30g croscarmellose sodium, 3.5g stearin Magnesium acid is mixed evenly and directly compressed into tablets to obtain cefixime tablets.
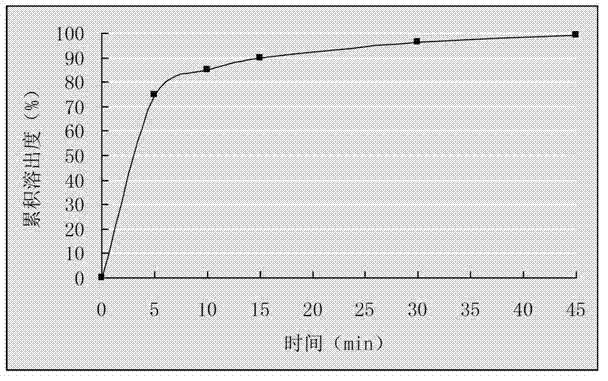
Embodiment 2
[0023] Dissolve 500g of cefixime and 1000g of lecithin in 10L of methanol, add 250g of silicon dioxide, and stir; evaporate the methanol to dryness under reduced pressure below 45°C, take out the evaporated compound, crush it through a 80-100 mesh sieve, and obtain Cefixime phospholipid complex of silicon dioxide; weigh 175g of cefixime phospholipid complex containing silicon dioxide, mix evenly with 160g microcrystalline cellulose, 28g crospovidone, 4.0g magnesium stearate and directly press Tablets, get cefixime tablets.
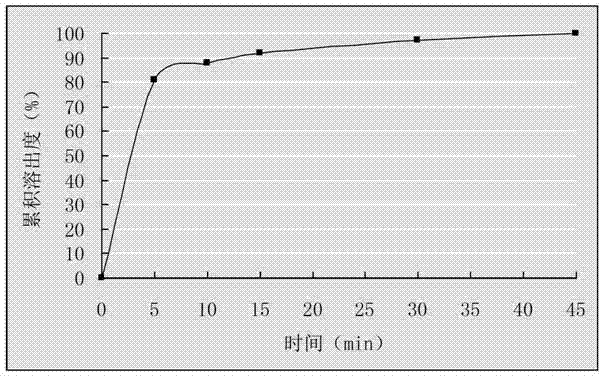
Embodiment 3
[0025] Dissolve 500g of cefixime and 1250g of distearoylphosphatidylcholine in 10L of methanol, add 200g of silicon dioxide, and stir; evaporate the methanol to dryness under reduced pressure below 50°C, take out the evaporated complex, and crush it over 80- 100 mesh sieves to obtain the cefixime phospholipid complex containing silicon dioxide; weigh 195g of the cefixime phospholipid complex containing silicon dioxide, mix it with 120g microcrystalline cellulose, 45g mannitol, and 30g low-substituted hydroxypropyl fiber Plain, 4.5g magnesium stearate mix evenly direct tabletting, get cefixime tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com