Aspartase-targeted activated adriamycin derivative as well as preparation method and application thereof
An aspartase target, adriamycin technology, applied in the field of antitumor drug compounds, can solve the problems of limited dose, toxic and side effects, and achieve the effects of improved drug efficacy, reduced toxicity, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
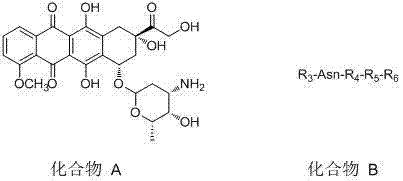
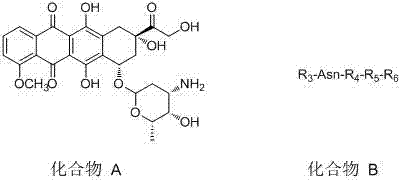
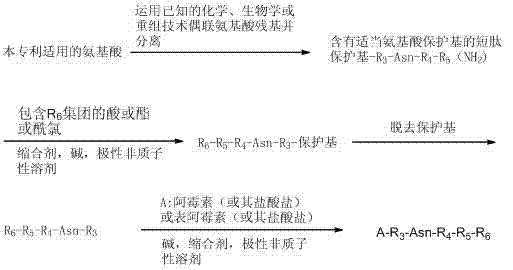
[0034] The invention provides a method for preparing a polypeptide doxorubicin activated by aspartase targeting tumor microenvironment, comprising the following steps: first, using known chemical, biological or recombinant techniques to couple amino acid residues, and separating The formed polypeptide R3-Asn-R4-R5 is obtained; secondly, the nitrogen segment of the formed polypeptide is formed into a covalent bond R3- Asn-R4-R5-R6; then the carboxyl group of R3 of R3-Asn-R4-R5-R6 is combined with doxorubicin or its salt or doxorubicin derivative and its salt (ie The amino group of compound A) is covalently bonded to form a doxorubicin analog with a short peptide and a group capable of binding to serum albumin, compound A—R3-Asn-R4-R5-R6. The reaction scheme is as follows:
[0035]
[0036] Among them, the condensing agent includes known chemical reagents used for the condensation reaction of carboxylic acid and amino group to form amides, used alone or in combination, such ...
Embodiment 1
[0039] Example 1: Synthesis of activated polypeptide doxorubicin S1 and S2 targeting the tumor microenvironment
[0040] The synthetic routes of S1 and S2 are as follows:
[0041] .
[0042] 1) Synthesis of Cbz-L-Ala-L-Ala-OMe (I)
[0043]
[0044] Dissolve N-benzyloxycarbonyl-L-alanine (100g, 0.45mol) in dry N,N-dimethylformamide (3L), add 1-hydroxybenzotriazole (72.6g , 0.54mol) and 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (103.3g, 0.54mol), after stirring for 1 hour, add dropwise in ice bath to 0°C L-alanine methyl ester (46.2g, 0.45mol) and N,N-diisopropylethylamine (173.8g, 1.34mol) in N,N-dimethylformamide (1L) solution, dropwise After completion, stir at room temperature for 10 hours, evaporate the solvent under reduced pressure, dissolve the crude product in dichloromethane (2L), wash with saturated ammonium chloride solution, water and saturated sodium chloride solution successively, and wash the organic phase with anhydrous sodium sulfate A...
Embodiment 2
[0081] Embodiment 2 obtains injection
[0082] The synthesized S1 and S2 were vacuum-dried to obtain a red powder, which was sterilized by gas for aseptic treatment and subpackaged in a sterile room. Before the animal experiment, dissolve it with water for injection containing 50% alcohol in a sterile room, and then dilute it with water for injection to the required concentration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com