Application of selective estrogen receptor modulator
A technology of selection and reaction, applied in the field of stilbene derivatives, can solve problems such as toxic and side effects, and achieve the effects of low cost, simple method operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
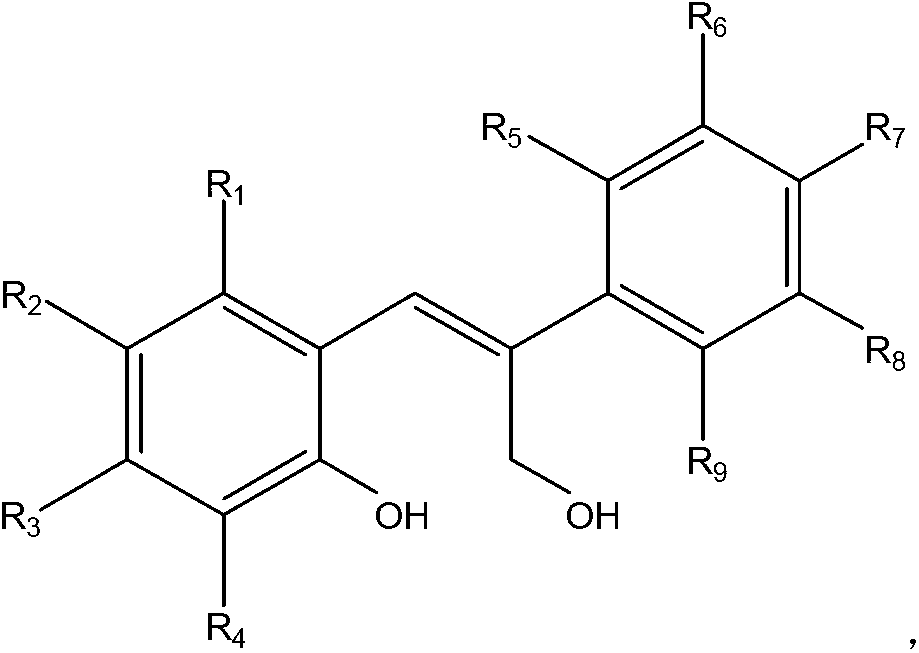
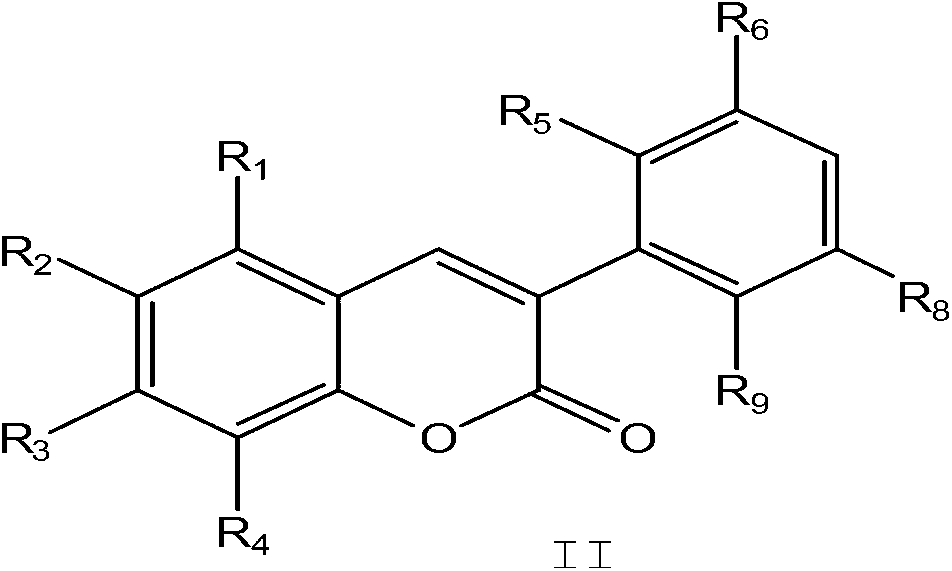
[0024] Step a) Synthesis of 3-(4-methoxyphenyl)coumarin IIa
[0025] Under nitrogen protection, 10 mmol of salicylaldehyde, 10 mmol of 4-methoxyphenylacetic acid, 10 mmol of potassium acetate, and 30 mL of acetic acid were sequentially added into the three-neck flask, and the reaction was refluxed for 3 hours. After the reaction, cool, neutralize, extract, collect the organic layer, and concentrate to obtain a light yellow solid powder, mp143-144°C.
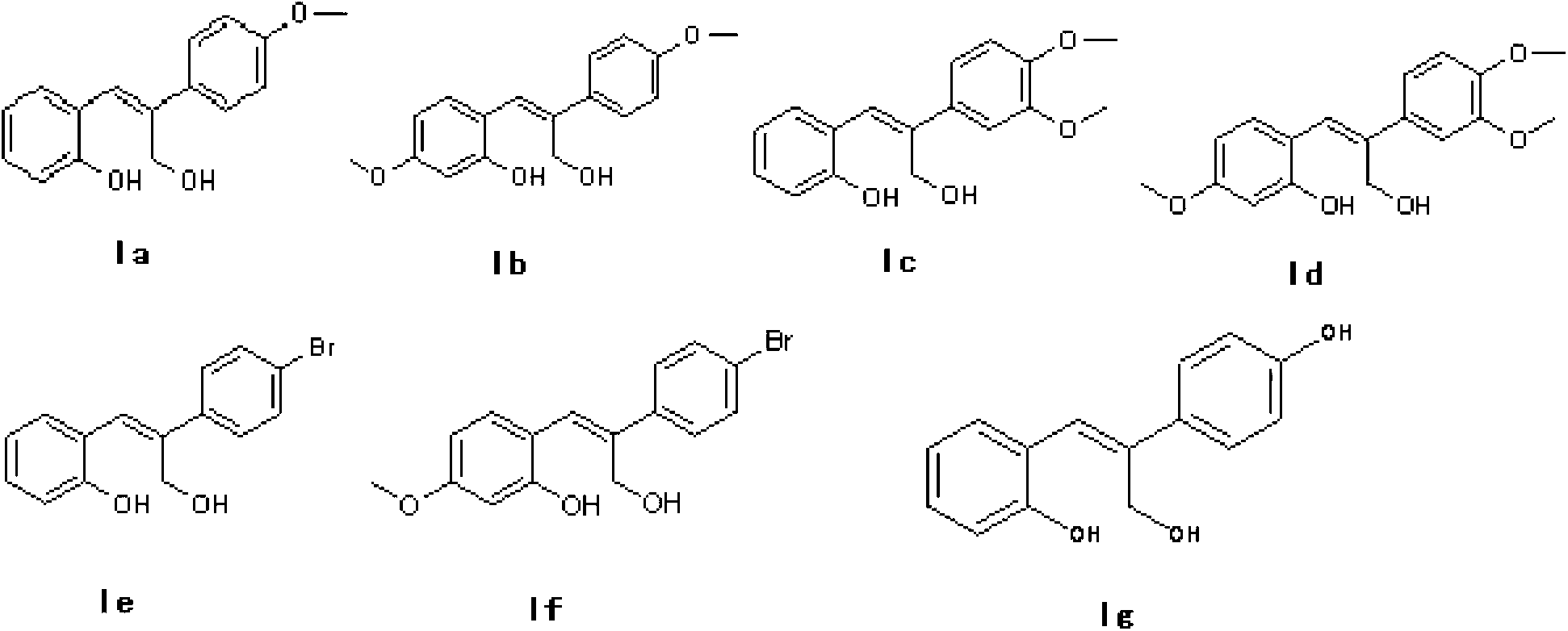
[0026] Step b) synthesis of target product Ia
[0027] The product of step a above was dissolved in anhydrous dichloromethane, and a certain amount of DIBAL-H solution was slowly added dropwise at -30°C. After dropping, the system naturally rose to room temperature, and stirred for 3 hours. After the reaction was completed, it was quenched and filtered to obtain the target product. White solid, mp124-125℃. 1 H NMR (400MHz, DMSO-d 6 )δ:7.57-7.55(2H,d,J=8.8Hz),7.46-7.44(1H,d,J=8.0Hz),7.11(1H,t,J=7.6Hz),6.94-6.91(3H,m ),6.87-6...
Embodiment 2
[0029] Step a) Synthesis of 7-methoxy-3-(4-methoxyphenyl)coumarin IIb
[0030] According to the method shown in step a of Example 1, using 4-methoxysalicylaldehyde and 4-methoxyphenylacetic acid as raw materials, the target product was synthesized, a white solid, mp186-187°C
[0031] Step b) synthesis of target product Ib
[0032] According to the method shown in step b of Example 1, IIb was placed in dichloromethane, using DIBALH as a reducing agent, and reacted at -15°C for 5h to obtain a white solid with a melting point of 128-129°C. 1 H NMR (400MHz, DMSO-d 6 )δ:7.52(2H,d,J=8.8Hz),7.40(1H,d,J=8.0Hz),6.92(2H,d,J=9.2Hz),6.84(1H,s),6.43-6.41( 2H,m), 4.39(2H,s), 3.75(3H,s), 3.70(3H,s). 13 C NMR (100MHz, DMSO-d 6 )δ: 160.1(s), 158.7(s), 156.9(s), 137.4(s), 134.5(s), 1301.0(s), 127.7(d), 124.4(s), 117.5(s), 114.0( d),104.7(s),101.4(s),59.1(s),55.3(d).IR(KBr)cm -1 :3494,3268,1610,1432,1232,1019,839,811.MS(EI)m / z(%):286(M+ ), 267, 253, 161, 137. HRMS (EI) Calcd for C17H18O4: ...
Embodiment 3
[0034] Step a) Synthesis of 3-(3,4-dimethoxyphenyl)coumarin IIc
[0035] According to the method shown in step a of Example 1, using salicylaldehyde and 3,4-dimethoxyphenylacetic acid as raw materials, the target product was synthesized, a white solid, mp122-123°C
[0036] Step b) synthesis of target product Ic
[0037] According to the method shown in step b of Example 1, IIb was placed in dichloromethane, using aluminum hydride as a reducing agent, and reacted at -20°C for 5h to obtain a white solid, mp154-156°C. 1 H NMR (400MHz, DMSO-d 6 )δ:7.44(1H,d,J=7.6Hz),7.16-7.08(3H,m),6.93(1H,d,J=8.2Hz),6.88(1H,s),6.84-6.78(2H,m ), 4.39(2H,s), 3.78(3H,s), 3.75(3H,s). 13 C NMR (100MHz, DMSO-d 6 )δ: 155.8(s), 148.9(s), 148.6(s), 139.2(s), 134.8(s), 130.5(s), 128.9(s), 125.0(s), 124.6(s), 119.2( s),119.1(s),115.7(s),112.0(s),110.7(s),58.8(s),55.9(d).IR(KBr)cm-1:3467,3214,1598,1456,1243 ,1004,897,860,803,762.MS(EI)m / z(%):286(M + ), 267, 237, 165, 151, 73, 57. HRMS (EI) Calcd for C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com