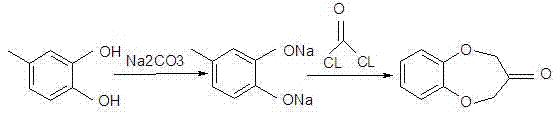
Synthesis method of watermelon ketone
A synthesis method and watermelon ketone technology are applied in the synthesis field of watermelon ketone, can solve the problems of low reaction yield, harsh reaction conditions, low yield and the like, and achieve the effects of simple temperature control, reduced production cost and high material selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In the 2000ml four-necked round bottom flask that thermometer, reflux condenser, water trap, agitator are equipped with, add mass concentration and be 50% sodium carbonate solution 440g, under nitrogen protection, add 124g4-methylcatechol, be warming up to React at 60-80°C for 1-2 hours, add 800ml of toluene to heat and evaporate to take away the moisture in the material;
[0028] When no moisture is evaporated out of the material, the temperature of the material is lowered to 60°C, 5g of triethylamine and 14g of ammonium iodide are added in sequence, and a mixed solution of 130g of 1,3-dichloroacetone and 300ml of ethanol is slowly added dropwise, and the temperature is continued after dropping Reflux reaction for 3-5 hours; during the reaction process, take samples for GC analysis, stop the reaction when the 1,3-dichloroacetone is less than 0.01%, cool down, filter with suction to obtain the crude product, then remove the ethanol and toluene from the crude product unde...
Embodiment 2
[0031] In a 2000ml four-necked round-bottomed flask equipped with a thermometer, a reflux condenser, a water separator, and an agitator, add a mass concentration of 460g of a 50% sodium carbonate solution, under nitrogen protection, add 124g of 4-methylcatechol, and control the temperature at 60 React at ~80°C for 1-2 hours, add 800ml of toluene to heat and evaporate to take away the moisture in the material;
[0032] When no moisture is evaporated out of the material, the temperature of the material is lowered to 60°C, .8g of diethylamine and 15g of ammonium iodide are added in sequence, and a mixed solution of 120g of 1,3-dichloroacetone and 300ml of butanone is slowly added dropwise. Afterwards, continue to insulate and reflux for 3-5 hours; during the reaction process, take samples for GC analysis, stop the reaction when the 1,3-dichloroacetone is less than 0.01%, cool, and filter the crude product, and then remove the butylated product from the crude product under negative...
Embodiment 3
[0035] In a 2000ml four-necked round-bottomed flask equipped with a thermometer, a reflux condenser, a water separator, and an agitator, add a mass concentration of 480g of a 50% sodium carbonate solution, nitrogen protection, add 124g of 4-methylcatechol, and control the temperature at 60 React at ~80°C for 1-2 hours, add 800ml of toluene to heat and evaporate to take away the moisture in the material;
[0036] When no moisture is evaporated out of the material, the temperature of the material is lowered to 60°C, 10g of triethylamine and 17g of ammonium iodide are added in sequence, and a mixed solution of 126g of 1,3-dichloroacetone and 500ml of butanone is slowly added dropwise. Continue heat preservation and reflux reaction for 3-5 hours; take samples for GC analysis during the reaction process, stop the reaction when the 1,3-dichloroacetone is less than 0.01%, cool, and filter the crude product, and then remove the butanone from the crude product under negative pressure ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com