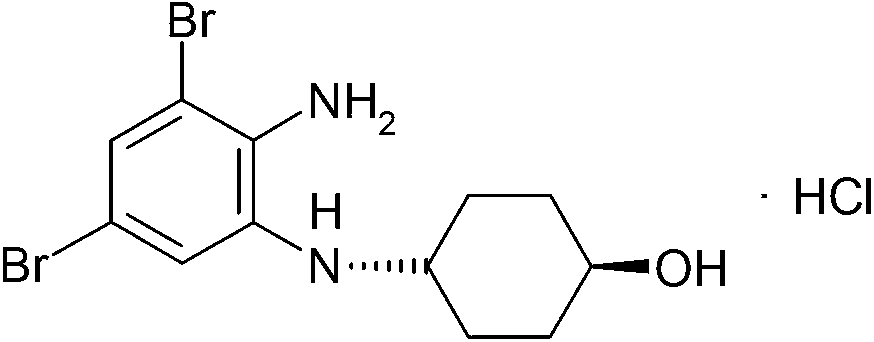
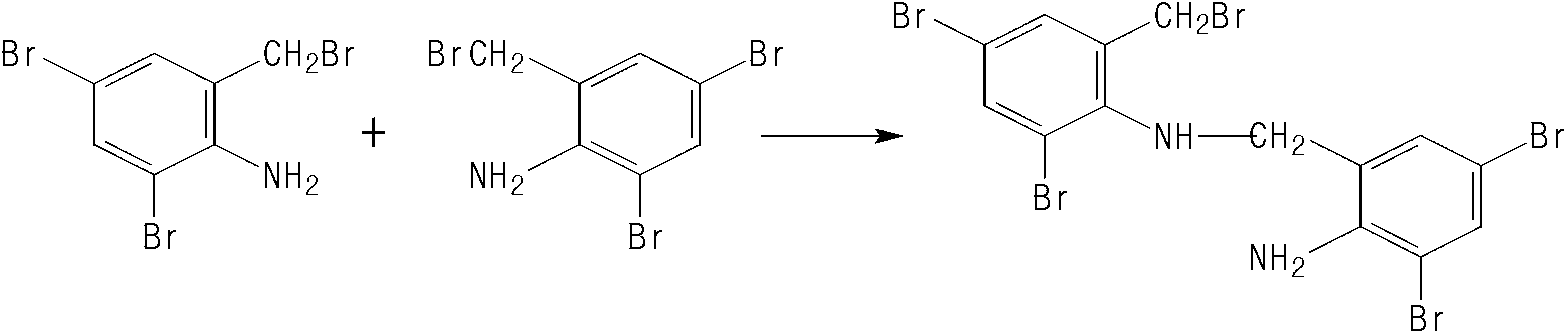
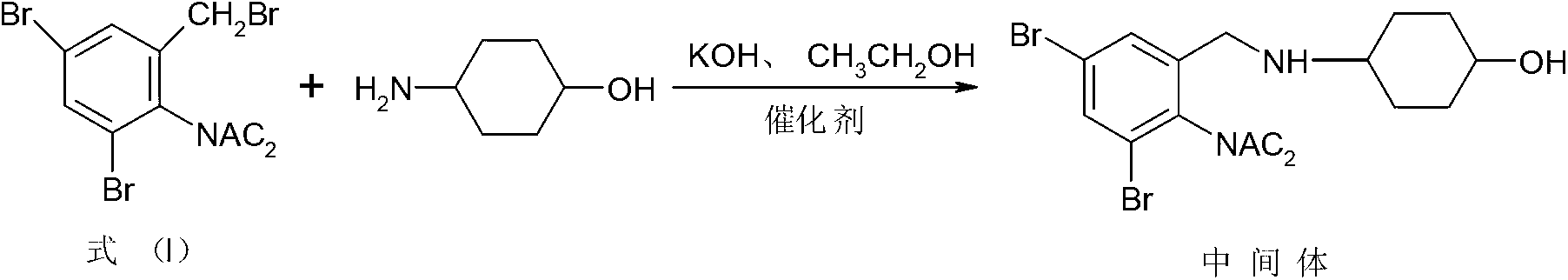
Synthesis method of ambroxol hydrochloride compound
A technology of ambroxol hydrochloride and a synthetic method, which is applied in the field of drug synthesis, can solve the problems that impurities cannot be effectively avoided, and the type and content of impurities cannot be effectively reduced, so as to improve the completeness of the reaction, improve the quality of the product, and increase the yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Synthesis of Ambroxol intermediate
[0051] Add 10g of p-aminocyclohexanol to a clean and anhydrous reaction bottle, add 180ml of absolute ethanol, stir quickly to dissolve, after dissolving, add 16.5g of potassium hydroxide, 0.1g of DMAP, 1.2g of 4-dimethylaminocyclohexanol in sequence , Control the temperature at 60°C and slowly add 40g of the compound of formula (I) in batches, continue to control the temperature at 60°C for 3 hours, after the reaction, control the temperature at 45°C to evaporate 110ml of absolute ethanol under reduced pressure, and control the time Slowly cool down to 5°C for 1 hour, grow crystals for 1 hour, filter with suction, and dry to obtain the intermediate of ambroxol, which is directly used in the next reaction without purification. The reaction formula is as follows:
[0052]
[0053] (2) Synthesis of ambroxol hydrochloride crude product
[0054] Add 160ml of ethanol, 65ml of water, 59ml of concentrated hydrochloric acid and 25ml...
Embodiment 2
[0057] (1) Synthesis of Ambroxol intermediate
[0058] Add 10g of p-aminocyclohexanol to a clean anhydrous reaction bottle, add 190ml of anhydrous methanol, stir and dissolve quickly, after dissolving, add 15g of potassium hydroxide, 0.05g of DMAP, and 1.0g of 4-dimethylaminocyclohexanol in sequence, Control the temperature at 75°C and slowly add 60g of the compound of formula (I) in batches, and continue to control the temperature at 75°C for 1 hour. The temperature was slowly lowered to 10°C within 1 hour, the crystal was grown for 3 hours, filtered with suction, and dried to obtain the intermediate of ambroxol, which was directly used in the next reaction without purification. Reaction formula is with embodiment 1.
[0059] (2) Synthesis of ambroxol hydrochloride crude product
[0060] Add 160ml of ethanol, 65ml of water, 36ml of concentrated hydrochloric acid and 25ml of glacial acetic acid into a clean and water-free reaction bottle, add 36g of ambroxol intermediate at ...
Embodiment 3
[0062] (1) Synthesis of Ambroxol intermediate
[0063] Add 10g of p-aminocyclohexanol to a clean anhydrous reaction bottle, add 180ml of absolute ethanol, stir quickly to dissolve, after dissolving, add 16.5g of potassium hydroxide, 0.1g of DMAP, 1.2g of 4-dimethylaminocyclohexanol in sequence , control the temperature at 70°C and slowly add 40g of the compound of formula (I) in batches, and continue to control the temperature at 70°C for 2 hours. Slowly cool down to 8°C for 1.5 hours, grow crystals for 2 hours, filter with suction, and dry to obtain the intermediate of ambroxol, which is directly used in the next reaction without purification. Reaction formula is with embodiment 1.
[0064] (2) Synthesis of ambroxol hydrochloride crude product
[0065] Add 160ml of ethanol, 65ml of water, 59ml of concentrated hydrochloric acid and 25ml of glacial acetic acid into a clean and water-free reaction bottle, add 36g of ambroxol intermediate at a controlled temperature of 48°C, st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com