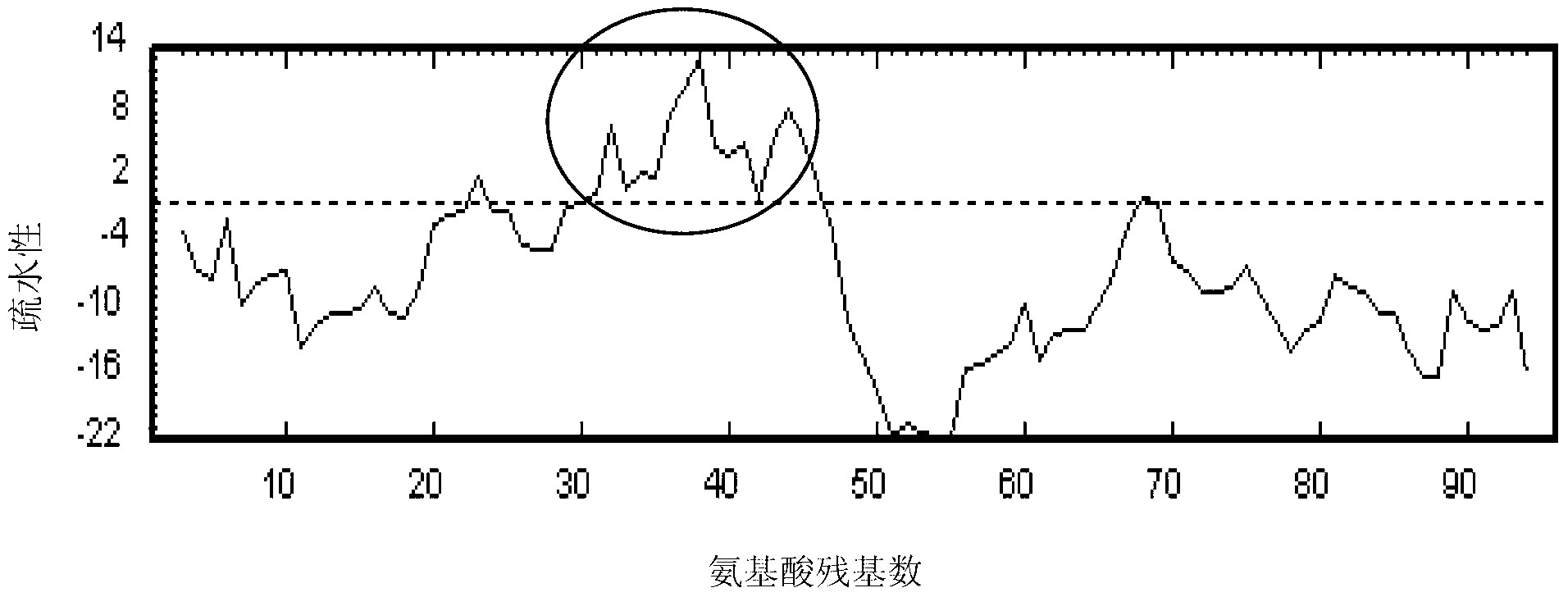
Hydrophobic-region-deletion HIV (Human Immunodeficiency Virus) type I Tat protein mutant sequence and applications thereof
A technology for human immunodeficiency and protein mutants, applied in applications, viral peptides, antiviral agents, etc., can solve the problems of low titer of immune protective antibodies and poor cross-reactivity, and achieve enhanced immune response and prokaryotic expression. Increased, strong immunogenic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Obtaining a Tat protein that lacks a hydrophobic region through the construction of a recombinant plasmid and prokaryotic expression
[0047] 1.1. In vitro synthesis of the target gene
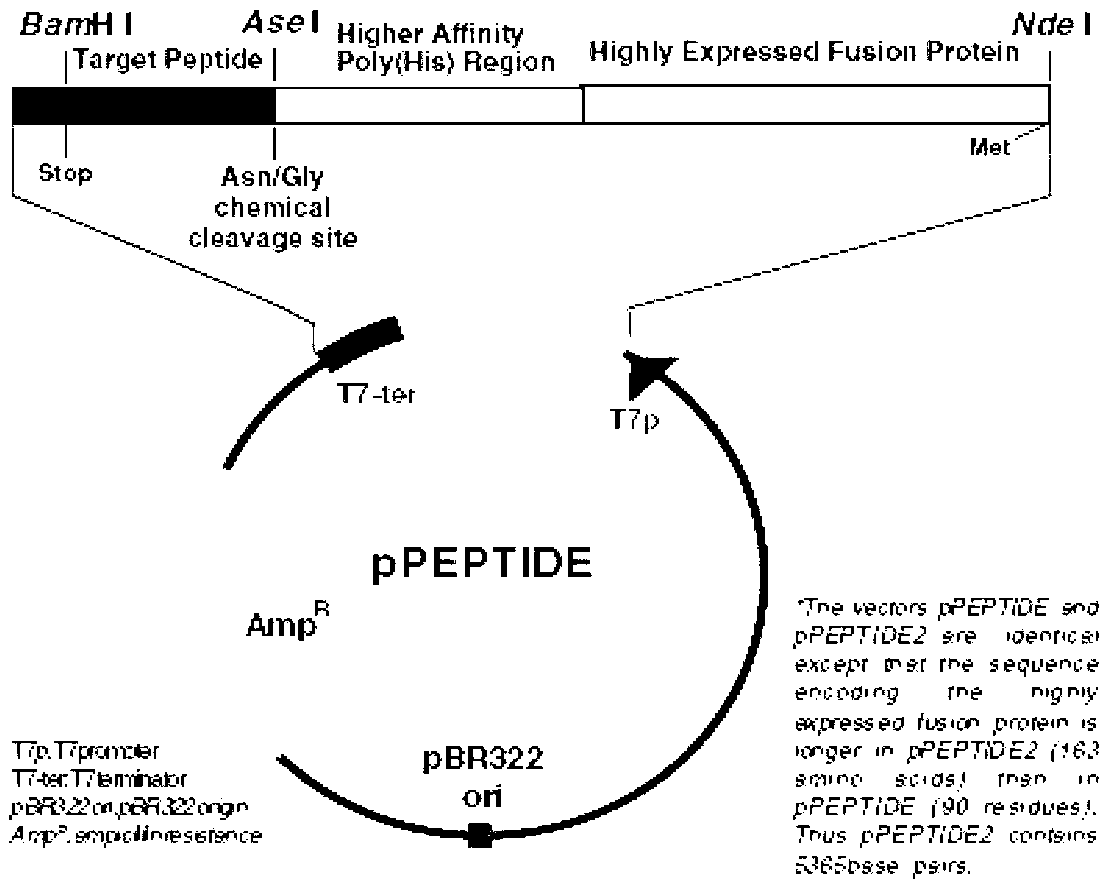
[0048] The tat1-101-HCVC122-191 DNA fragment was synthesized by the method of Overlap PCR, and the PCR product was recovered by the gel recovery kit, digested by Ase I and BamH I two restriction endonucleases, and the target fragment was recovered, and then mixed with the same The pPEPTIDE (purchased from TAKARA company) vector after Ase I and BamH I digestion ( figure 2 ) connection, and the connection product was transformed into E.coli Top10 competent cells. The plasmid of the transformed colony of the single clone was extracted and sequenced, and the recombinant plasmid with correct sequencing was named pPEP-Tat1-101-HCVC(122-191).
[0049]DNA fragments encoding Tat1-31 and Tat46-101-HCVC (122-191) peptides were synthesized in vitro by PCR method. The PCR reaction sys...
Embodiment 2
[0060] Example 2: The Tat protein gold-labeled immune complex was obtained by preparation and labeling, and its quality was identified.
[0061] 2.1. Preparation of colloidal gold
[0062] Heat 100ml of 0.01% chloroauric acid solution to boiling, accurately add 0.7ml of 1% trisodium citrate under stirring at 200rpm, and the golden yellow chloroauric acid solution turns purple. Continue heating and stirring for 15 minutes, and then dilute to 100ml after cooling.
[0063] 2.2. Electron microscope detection of colloidal gold particle size and uniformity
[0064] The size and uniformity of colloidal gold particles were detected by transmission electron microscopy, and it was found that the average particle diameter of colloidal gold was 75nm, and the uniformity was good ( Figure 7 ).
[0065] 2.3. Tat△(31-45)-PET protein-labeled colloidal gold solution
[0066]The purified Tat(△31-45)-PET protein was dialyzed against 0.005Mol / L pH 9.0NaCl solution at 4°C for 9 hours to determ...
Embodiment 3
[0071] Example 3: Detection of changes in the immunogenicity of Tat mutants and the impact of colloidal gold labeling on its epitope display through animal immunity tests
[0072] 3.1. Animal immunization experiments
[0073] Take 100 μl of purified full-length Tat-PET, Tat(△31-45)-PET fusion protein and Tat(△31-45)-PET protein gold standard (both protein content is 0.01mg) and Freund’s After mixing equal volumes of the complete adjuvant, subcutaneously inject immunized healthy female six-week-old C57BL mice (Experimental Animal Center, Second Military Medical University) into the paws and abdomen, with 5 experimental mice in each group; after that, a booster immunization is performed every 2 weeks. Incomplete Freund's adjuvant was used for immunization, and a total of 3 booster immunizations were performed, and the method and dose were the same as those of the initial immunization. At the same time, colloidal gold and normal saline control groups were set up. Two weeks afte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com