Gradient coated LiNiO2 material and preparation method
A lithium nickelate and gradient technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of rapid capacity decay and poor thermodynamic stability of lithium nickelate, improve cycle performance and thermodynamic stability, solve instability, good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
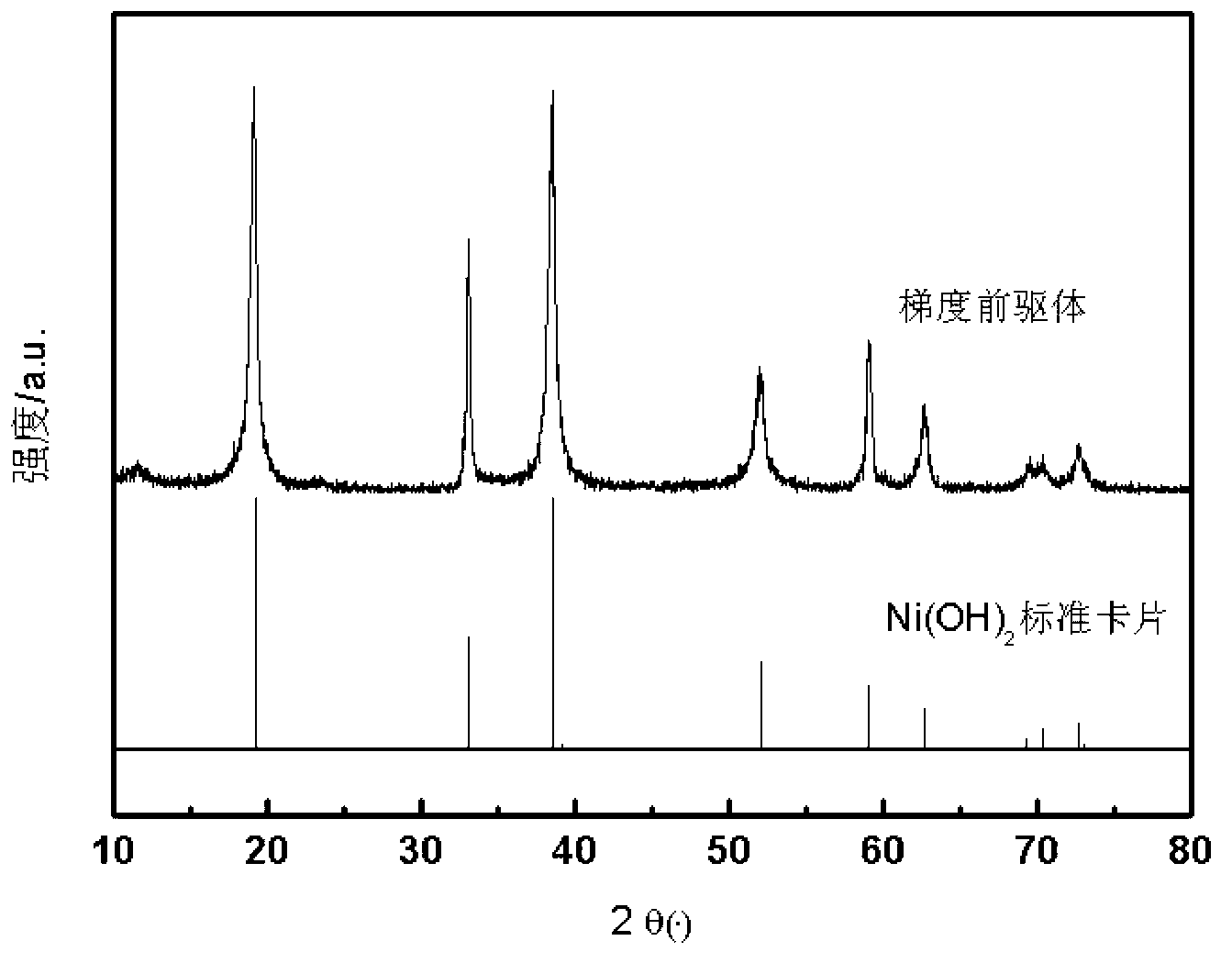
[0047] Prepare 0.5mol / L nickel sulfate solution, 0.8mol / L cobalt manganese sulfate solution (cobalt manganese ratio is 1:1), 3mol L -1 sodium hydroxide solution and 0.5mol L -1 of ammonia solution. The three solutions of nickel sulfate, sodium hydroxide and ammonia water were simultaneously fed into the reaction kettle through the dosage pump at a flow rate of 1.5 L / h by a peristaltic pump, and the reaction temperature was kept at 30°C with continuous stirring, while the reaction was controlled at pH=12. There is a large amount of green Ni(OH) at the beginning of the reaction 2 Precipitation formed. The reaction lasts for a period of time until 90% NiSO 4 After the solution is consumed, the cobalt-manganese sulfate mixed ion solution is continuously input into the nickel sulfate solution, and stirred and mixed. Then the formed new mixed ion solution is continuously input into the reactor to continue the precipitation. After the dropwise addition of the mixed solution was ...
Embodiment 2
[0049] First prepare 1mol / L nickel sulfate solution, 0.7mol / L cobalt manganese aluminum sulfate solution (cobalt manganese aluminum ratio is 1:1:1:0.5), 1mol L -1 sodium hydroxide solution and 0.5mol L -1 of ammonia solution. The three solutions of nickel sulfate, sodium hydroxide and ammonia water were simultaneously fed into the reactor by a peristaltic pump at a flow rate of 1.5 L / h, and the reaction temperature was kept at 40°C with continuous stirring, while the reaction was controlled at pH=11. There is a large amount of green Ni(OH) at the beginning of the reaction 2 Precipitation formed. The reaction continues for a period of time until 70% NiSO 4 After the solution is consumed, the mixed ion solution of cobalt, manganese and aluminum sulfate is continuously input into the nickel sulfate solution, and stirred and mixed. At the same time, the formed new mixed ion solution is continuously input into the reactor to continue the precipitation. After the dropwise addit...
Embodiment 3
[0051] First prepare 2mol / L nickel sulfate solution, 0.5mol / L cobalt manganese sulfate solution (cobalt manganese ratio is 1:2), 1mol L -1 sodium hydroxide solution and 0.1mol L -1 of ammonia solution. The three solutions of nickel sulfate, sodium hydroxide and ammonia water were simultaneously input into the reaction kettle through the dosage pump at a flow rate of 1.5 L / h by a peristaltic pump, and the reaction temperature was kept at 30°C with continuous stirring, while the reaction was controlled at pH=10. There is a large amount of green Ni(OH) at the beginning of the reaction 2 Precipitation formed. The reaction continues for a period of time until 50% NiSO 4 After the solution is consumed, the cobalt-manganese sulfate mixed ion solution is continuously input into the nickel sulfate solution, and stirred and mixed. At the same time, the formed new mixed ion solution is continuously input into the reactor to continue the precipitation. After the dropwise addition of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com