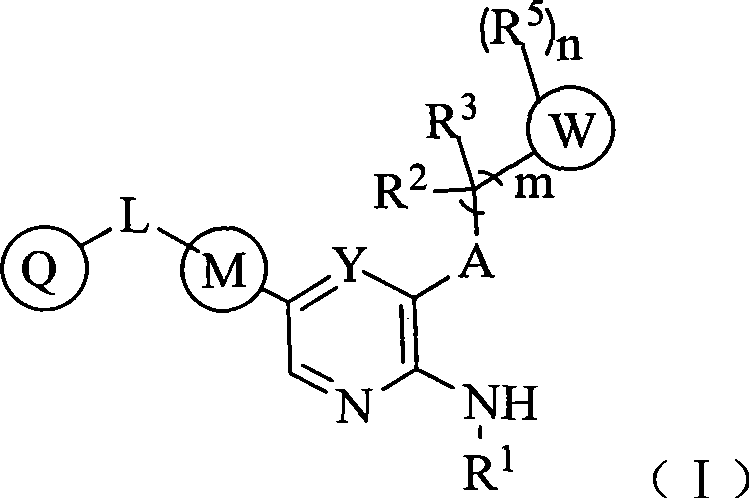
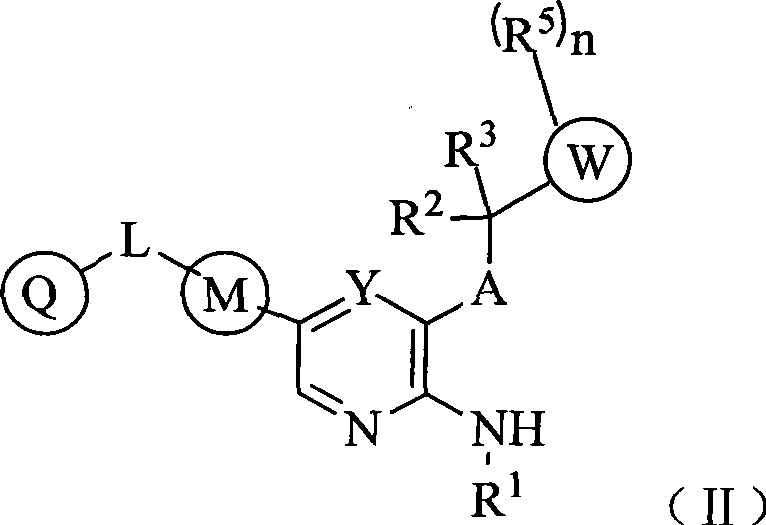
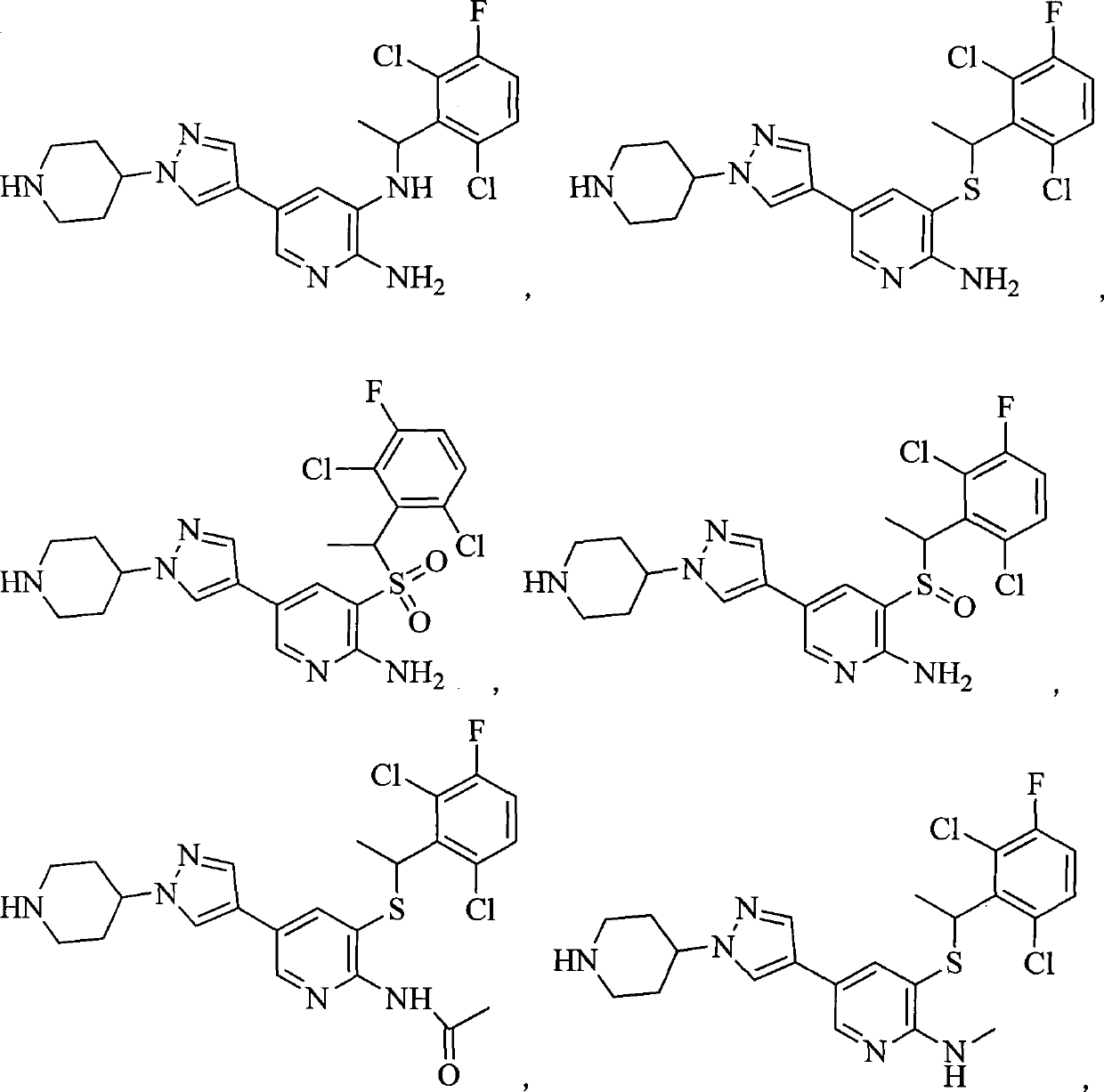
Aryl kinase inhibitor
An alkyl and amino technology, applied in the field of aryl kinase inhibitors, can solve problems such as malignant transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0130] The present invention also provides the preparation method of above-mentioned compound, but not limited to following method, and reaction equation is as follows:
[0131] when for hour,
[0132]
[0133] Reaction steps:
[0134] Step 1 Preparation of intermediate 1 and intermediate 2
[0135] Prepared with reference to US2008 / 300273A1 literature.
[0136] The preparation of step 2 intermediate 3
[0137] Dissolve intermediate 1 and an equivalent amount of intermediate 2 in an appropriate amount of N, N-dimethylformamide, and add catalyst Pd (PPh 3 ) 4 1. Organic solvent (such as ethanol and toluene, water), react at 80°C until the raw materials disappear, add water after cooling down, then extract, filter with suction, and use HPLC to separate and purify to obtain intermediate 3.
[0138] Step 3 Preparation of Compound I' of the present invention
[0139] Intermediate 3 was dissolved in dioxane of saturated hydrochloric acid, stirred for 1 hour, the solvent...
Embodiment 1N
[0168] Example 1N 3 -(1-(2,6-dichloro-3-fluorophenyl)ethyl)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridine-2,3 - diamine (Compound 1) Preparation of Hydrochloride
[0169] (1) tert-butyl 4-hydroxypiperidine-1-carboxylate
[0170]
[0171] Dissolve tert-butyl 4-oxopiperidine-1-formyl ester (19.9g, 99.9mmol) in methanol (1000mL), cool in an ice-water bath, add sodium borohydride (5.7g, 0.15mol), and Stir for 10 hours, remove methanol by rotary evaporation, add water (250 mL), extract with ethyl acetate, concentrate and dry under reduced pressure to obtain the product (20 g, yield 99%).
[0172] (2) tert-butyl 4-(methylsulfonyloxy)piperidine-1-carboxylate
[0173]
[0174] 4-Hydroxypiperidine-1-carboxylic acid tert-butyl ester (20.1 g, 0.1 mol) and N, N-diisopropylethylamine (15 mL) were dissolved in dichloromethane, and methanesulfonyl chloride ( 17.1 g, 0.15 mol), stirred at room temperature for 3 hours. After the reaction was completed, it was filtered, and ...
Embodiment 23
[0198] Example 23-(1-(2,6-dichloro-3-fluorophenyl)ethylthio)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridine -2-amine ( Compound 2) Preparation
[0199] (1) 1-(2,6-dichloro-3-fluorophenyl)ethanol
[0200]
[0201] Dissolve 1-(2,6-dichloro-3-fluorophenyl)ethanone (20.7g, 0.1mol) in methanol (1000mL), cool in an ice-water bath, add sodium borohydride (5.7g, 0.15mol), Stir for 10 hours, remove the organic solvent by rotary evaporation, add water, extract with ethyl acetate, dry over anhydrous sodium sulfate, and remove the organic solvent by rotary evaporation (20.9 g, yield 100%).
[0202] (2) (2-(1-bromoethyl)-1,3-dichloro-4-fluorobenzene
[0203]
[0204] 1-(2,6-dichloro-3-fluorophenyl)ethanol (20.9g, 0.1mol) was dissolved in hydrobromic acid (100mL), refluxed for 3 hours, cooled to room temperature, extracted with ethyl acetate, and the combined The organic phase was washed with water, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com