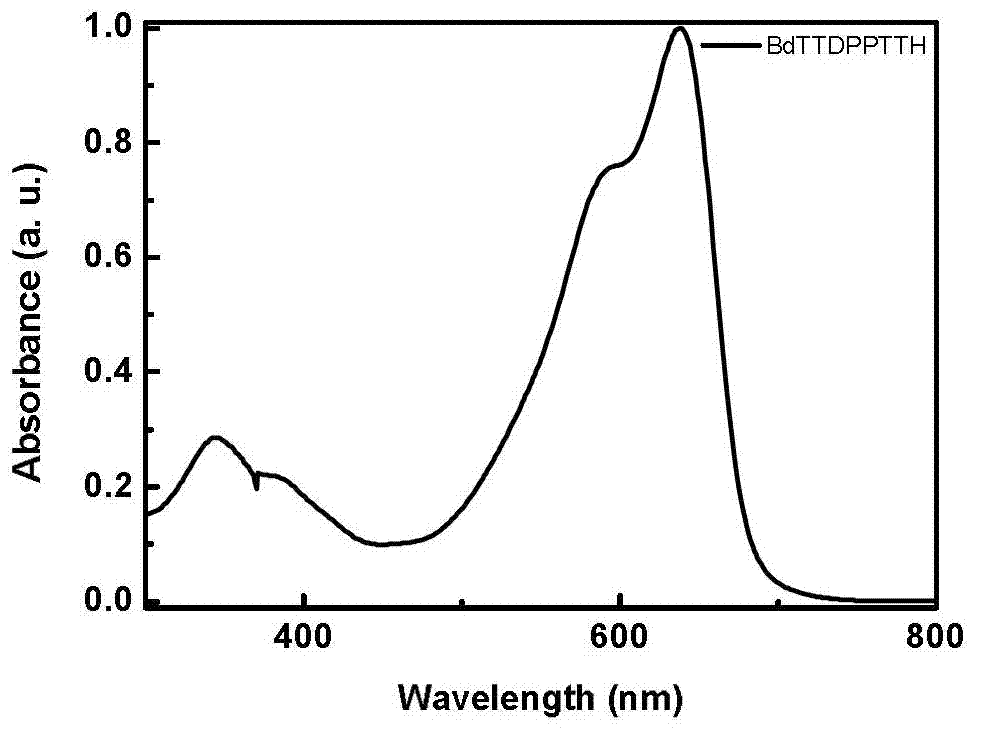
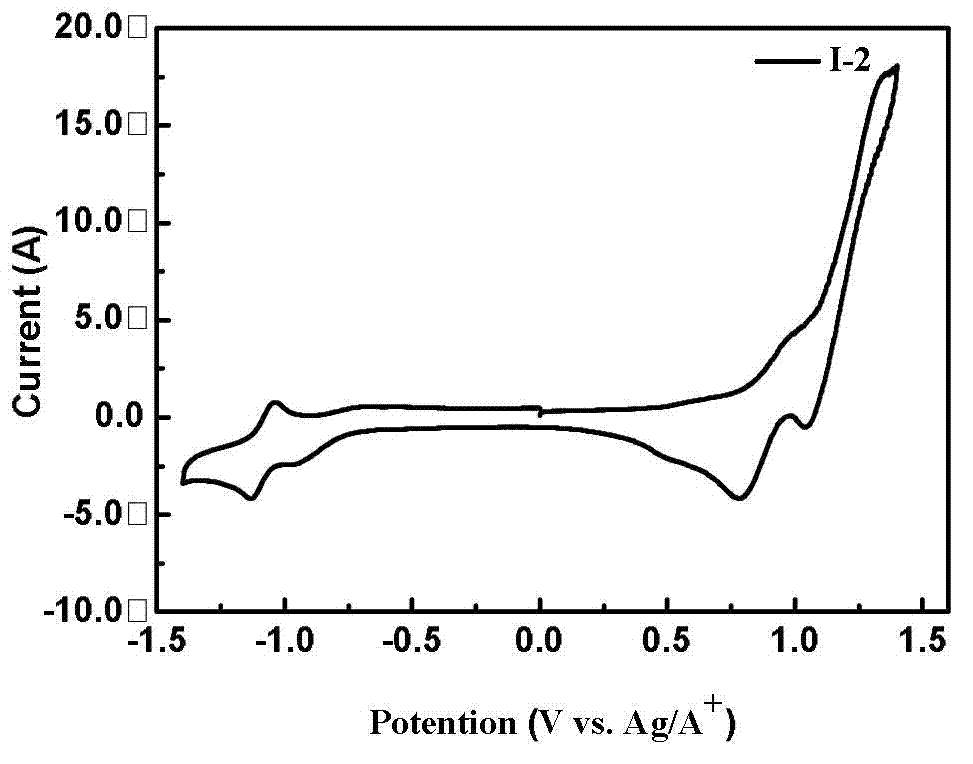
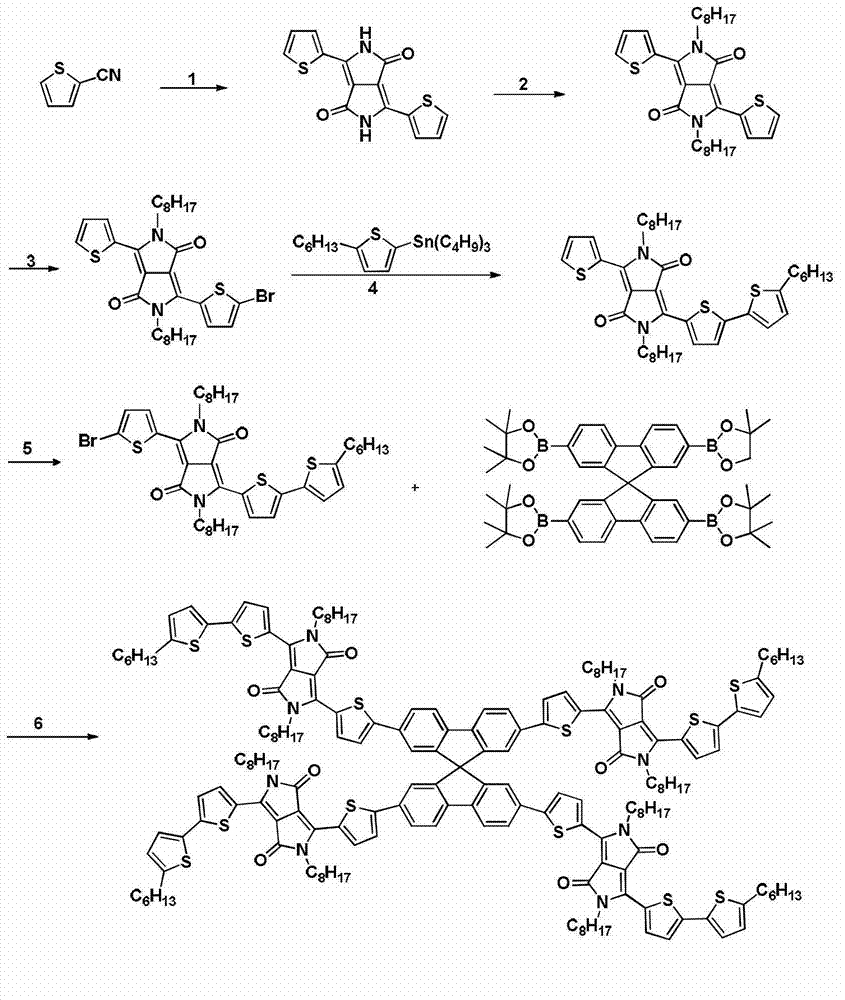
Diketopyrrolopyrrole derivative and preparation method and application thereof
A technology of diketopyrrolopyrrole and derivatives is applied in the field of solar cell materials to achieve the effects of satisfying usage requirements, easy preparation method and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of Compound (II): Under argon protection, potassium tert-butoxide (20g, 180mmol) was added to a 250mL round-bottomed flask, and injected into a solution of 2-cyanothiophene (16.4g, 150mmol) in tert-amyl alcohol (125mL) , the reaction mixture was heated to 100-110° C., dimethyl succinate (7.30 g, 50 mmol) was dissolved in tert-amyl alcohol (40 mL), and added dropwise to the reaction solution. The reaction was kept at the same temperature, stirred for 1 h, and then the by-product methanol was distilled off, and the reaction was continued at 100-110° C. for 2 h. The reaction mixture was cooled to 65°C, diluted with 50 mL of methanol, neutralized with acetic acid, and then refluxed for 10 minutes. The reaction was stopped, filtered, and the obtained black solid was washed twice with hot methanol and water, and dried in a vacuum oven to obtain compound (II) (10.6 g, 72%).
Embodiment 2
[0068] Synthesis of compound (III): in a 250ml two-necked flask, under argon protection, compound (II) (13.0g, 43.3mmol) and anhydrous potassium carbonate (24g, 173mmol) were dissolved in 250mLN, N-dimethylformamide , heated to 145°C, injected octyl bromide (38.6g, 200mmol), and stirred at 145°C for 12h. Stop the reaction, cool to room temperature, pour the reaction solution into 500 mL of ice water, filter, wash the solid several times with water and methanol, and dry in a vacuum oven to obtain a crude product that is separated by silica gel column chromatography (dichloromethane as eluent) , to obtain dark red product compound (III) (18.18 g, 80%). 1 H NMR (400MHz, CDCl 3 ): δ8.94(dd, 2H), 7.65(dd, 2H), 7.29(dd, 2H), 4.08(t, 4H), 1.75(m, 4H), 1.18-1.48(m, 20H), 0.88( t, 6H).
Embodiment 3
[0070] Synthesis of Compound (IV): Compound (III) (5.25g, 10mmol) was dissolved in 200mL of chloroform, N-bromosuccinimide (NBS) (1.87g, 10.5mmol) was added in portions at room temperature, React at room temperature overnight in the dark. Pour the reaction solution into 200 mL of water, extract with chloroform, wash with water, combine the organic phases, dry over anhydrous magnesium sulfate, spin off the solvent to obtain a crude product, recrystallize from chloroform and ethanol to obtain a purple-black solid (3.80 g, 63%) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com